Protein–energy malnutrition
| Protein–energy malnutrition | |
|---|---|
| Other names: Protein–calorie malnutrition, PEM, PCM | |
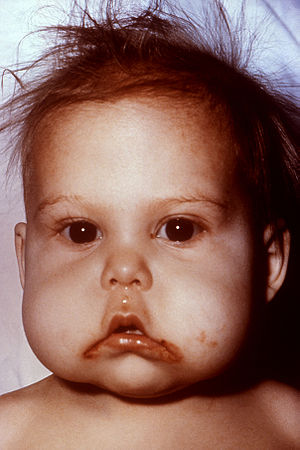 | |
| Child in the United States with signs of kwashiorkor, an example of protein-energy malnutrition. | |
Protein–energy malnutrition (PEM), sometimes called protein-energy undernutrition (PEU), is a form of malnutrition that is defined as a range of conditions arising from coincident lack of dietary protein and/or energy (calories) in varying proportions. The condition has mild, moderate, and severe degrees.
Types include:[1]
- Kwashiorkor (protein malnutrition predominant)
- Marasmus (deficiency in calorie intake)
- Marasmic kwashiorkor (marked protein deficiency and marked calorie insufficiency signs present, sometimes referred to as the most severe form of malnutrition)
PEM is fairly common worldwide in both children and adults and accounts for about 250 000 deaths annually.[2] In the industrialized world, PEM is predominantly seen in hospitals, is associated with disease, or is often found in the elderly.[3]
Note that PEM may be secondary to other conditions such as chronic renal disease[4] or cancer cachexia[5] in which protein energy wasting may occur.
Protein–energy malnutrition affects children the most because they have less protein intake.[further explanation needed] The few rare cases found in the developed world are almost entirely found in small children as a result of fad diets, or ignorance of the nutritional needs of children, particularly in cases of milk allergy.[6]
Prenatal protein malnutrition
Protein malnutrition is detrimental at any point in life, but protein malnutrition prenatally has been shown to have significant lifelong effects. During pregnancy, one should aim for a diet that consists of at least 20% protein for the health of the fetus[citation needed]. Diets that consist of less than 6% protein in utero have been linked with many deficits, including decreased brain weight, increased obesity, and impaired communication within the brain in some animals. Even diets of mild protein malnutrition (7.2%) have been shown to have lasting and significant effects in rats. The following are some studies in which prenatal protein deficiency has been shown to have unfavorable consequences.
- Decreased brain size: Protein deficiency has been shown to affect the size and composition of brains in rhesus monkeys. Monkeys whose mother had eaten a diet with an adequate amount of protein were shown to have no deficit in brain size or composition, even when their body weight amounted to less than one-half of that of the controls, whereas monkeys whose mothers had eaten low-protein diets were shown to have smaller brains regardless of the diet given after birth.[7]
- Impaired neocortical long-term potentiation: Mild protein deficiency (in which 7.2% of the diet consists of protein) in rats has been shown to impair entorhinal cortex plasticity (visuospatial memory), noradrenergic function in the neocortex, and neocortical long-term potentiation.[8]
- Altered fat distribution: Protein undernutrition can have varying effects depending on the period of fetal life during which the malnutrition occurred. Although there were not significant differences in the food intake, there were increased amounts of perirenal fat in rats that were protein-deprived during early (gestation days 0–7) and mid (gestation days 8–14) pregnancy, and throughout pregnancy, whereas rats that were protein-deprived only late in gestation (gestation days 15–22) were shown to have increased gonadal fat.[9]
- Increased obesity: Mice exposed to a low-protein diet prenatally weighed 40% less than the control group at birth (intrauterine growth retardation). When fed a high-fat diet after birth, the prenatally undernourished mice were shown to have increased body weight and adiposity (body fat), while those who were adequately nourished prenatally did not show an increase in body weight or adiposity when fed the same high-fat diet after birth.[10]
- Decreased birth weight, and gestation duration: Supplementation of protein and energy can lead to increased duration of gestation and higher birth weight. When fed a supplement containing protein, energy, and micronutrients, pregnant women showed more successful results during birth, including high birth weights, longer gestations, and fewer pre-term births, than women who had consumed a supplement with micronutrients and low energy but no protein (although this finding may be due to the increase of energy in the supplements, not the increase of protein).[11]
- Increased stress sensitivity: Male offspring of pregnant rats fed low-protein diets have been shown to exhibit blood pressure that is hyperresponsive to stress and salt.[12]
- Decreased sperm quality: A low-protein diet during gestation in rats has been shown to affect the sperm quality of the male offspring in adulthood. The protein deficiency appeared to reduce sertoli cell number, sperm motility, and sperm count.[13]
- Altered cardiac energy metabolism: Prenatal nutrition, specifically protein nutrition, may affect the regulation of cardiac energy metabolism through changes in specific genes.[14]
- Increased passive stiffness: Intrauterine undernutrition was shown to increase passive stiffness in skeletal muscles in rats.[15]
From these studies it is possible to conclude that prenatal protein nutrition is vital to the development of the fetus, especially the brain, the susceptibility to diseases in adulthood, and even gene expression. When pregnant females of various species were given low-protein diets, the offspring were shown to have many deficits. These findings highlight the great significance of adequate protein in the prenatal diet.
Epidemiology


Although protein energy malnutrition is more common in low-income countries, children from higher-income countries are also affected, including children from large urban areas in low socioeconomic neighborhoods. This may also occur in children with chronic diseases, and children who are institutionalized or hospitalized for a different diagnosis. Risk factors include a primary diagnosis of intellectual disability, cystic fibrosis, malignancy, cardiovascular disease, end stage renal disease, oncologic disease, genetic disease, neurological disease, multiple diagnoses, or prolonged hospitalization. In these conditions, the challenging nutritional management may get overlooked and underestimated, resulting in an impairment of the chances for recovery and the worsening of the situation.[16]
PEM is fairly common worldwide in both children and adults and accounts for 250 000 deaths annually.[3] In the industrialized world, PEM is predominantly seen in hospitals, is associated with disease, or is often found in the elderly.[3]
Co-morbidity
A large percentage of children that suffer from PEM also have other co-morbid conditions. The most common co-morbidities are diarrhea (72.2% of a sample of 66 subjects) and malaria (43.3%). However, a variety of other conditions have been observed with PEM, including sepsis, severe anaemia, bronchopneumonia, HIV, tuberculosis, scabies, chronic suppurative otitis media, rickets, and keratomalacia. These co-morbidities tax already malnourished children and may prolong hospital stays initially for PEM and may increase the likelihood of death.[17]
The general explanation of increased infectious comorbidity in malnourished people is that (1) the immune system is what prevents such diseases from being more widespread in healthy, well-nourished people and (2) malnutrition stresses and diminishes immune function. In other words, malnutrition tends to cause (mild or moderate) immunodeficiency, eroding the barriers that normally keep infectious diseases at bay. For example, this reversal is well established regarding the variable natural history of tuberculosis in the pre–TB drug era. Epidemiologically, there are also associations between malnutrition and other health risks via the common underlying factor of poverty. For example, condoms can reduce spread of HIV, but impoverished people often may not have money to buy condoms or a nearby place to buy them. Also, once a poor person has any particular infection, they may not have access to optimal treatment of it, which allows it to get worse, present more chances of transmission, and so on. Even when a developing country nominally/officially has national health insurance with universal health care, the poorest quarter of its population may face a de facto reality of poor health care access.
References
- ↑ Franco, V.; Hotta, JK; Jorge, SM; Dos Santos, JE (1999). "Plasma fatty acids in children with grade III protein–energy malnutrition in its different clinical forms: Marasmus, marasmic kwashiorkor, and kwashiorkor". Journal of Tropical Pediatrics. 45 (2): 71–5. doi:10.1093/tropej/45.2.71. PMID 10341499.
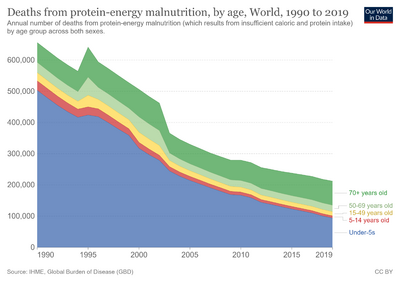
- ↑ "Deaths from protein-energy malnutrition, by age - Our World in Data". Archived from the original on 2022-03-26. Retrieved 2022-03-21.
- ↑ 3.0 3.1 3.2 "Dietary Reference Intake: The Essential Guide to Nutrient Requirements" published by the Institute of Medicine and available online at https://www.nap.edu/read/11537/chapter/14?term=protein-energy+malnutrition#151 Archived 2020-10-20 at the Wayback Machine
- ↑ Muscaritoli, Maurizio; Molfino, Alessio; Bollea, Maria Rosa; Fanelli, Filippo Rossi (2009). "Malnutrition and wasting in renal disease". Current Opinion in Clinical Nutrition and Metabolic Care. 12 (4): 378–83. doi:10.1097/MCO.0b013e32832c7ae1. PMID 19474712. S2CID 32472183.
- ↑ Bosaeus, Ingvar (2008). "Nutritional support in multimodal therapy for cancer cachexia". Supportive Care in Cancer. 16 (5): 447–51. doi:10.1007/s00520-007-0388-7. PMID 18196284. S2CID 7078558.
- ↑ Liu, T; Howard, RM; Mancini, AJ; Weston, WL; Paller, AS; Drolet, BA; Esterly, NB; Levy, ML; et al. (2001). "Kwashiorkor in the United States: Fad diets, perceived and true milk allergy, and nutritional ignorance". Archives of Dermatology. 137 (5): 630–6. PMID 11346341.
- ↑ Portman OW, Neuringer M, Alexander M (November 1987). "Effects of maternal and long-term postnatal protein malnutrition on brain size and composition in rhesus monkeys". The Journal of Nutrition. 117 (11): 1844–51. doi:10.1093/jn/117.11.1844. PMID 3681475. Archived from the original on 2020-07-03. Retrieved 2022-03-21.
- ↑ Hernández A, Burgos H, Mondaca M, Barra R, Núñez H, Pérez H, Soto-Moyano R, Sierralta W, Fernández V, Olivares R, Valladares L (2008). "Effect of prenatal protein malnutrition on long-term potentiation and BDNF protein expression in the rat entorhinal cortex after neocortical and hippocampal tetanization". Neural Plasticity. 2008: 1–9. doi:10.1155/2008/646919. PMC 2442167. PMID 18604298.
- ↑ Bellinger L, Sculley DV, Langley-Evans SC (May 2006). "Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats". International Journal of Obesity. 30 (5): 729–38. doi:10.1038/sj.ijo.0803205. PMC 1865484. PMID 16404403.
- ↑ Sutton GM, Centanni AV, Butler AA (April 2010). "Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity". Endocrinology. 151 (4): 1570–80. doi:10.1210/en.2009-1133. PMC 2850243. PMID 20160133. Archived from the original on 2020-07-03. Retrieved 2022-03-21.
- ↑ Rasmussen KM, Habicht JP (February 2010). "Maternal supplementation differentially affects the mother and newborn". The Journal of Nutrition. 140 (2): 402–6. doi:10.3945/jn.109.114488. PMID 20032480. Archived from the original on 2020-07-03. Retrieved 2022-03-21.
- ↑ Augustyniak RA, Singh K, Zeldes D, Singh M, Rossi NF (May 2010). "Maternal protein restriction leads to hyperresponsiveness to stress and salt-sensitive hypertension in male offspring". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 298 (5): R1375–82. doi:10.1152/ajpregu.00848.2009. PMC 2867525. PMID 20200128.
- ↑ Toledo FC, Perobelli JE, Pedrosa FP, Anselmo-Franci JA, Kempinas WD (2011). "In utero protein restriction causes growth delay and alters sperm parameters in adult male rats". Reproductive Biology and Endocrinology. 9: 94. doi:10.1186/1477-7827-9-94. PMC 3141647. PMID 21702915.
- ↑ Slater-Jefferies JL, Lillycrop KA, Townsend PA, Torrens C, Hoile SP, Hanson MA, Burdge GC (August 2011). "Feeding a protein-restricted diet during pregnancy induces altered epigenetic regulation of peroxisomal proliferator-activated receptor-α in the heart of the offspring". Journal of Developmental Origins of Health and Disease. 2 (4): 250–255. doi:10.1017/S2040174410000425. PMC 3191520. PMID 22003431.
- ↑ Toscano AE, Ferraz KM, Castro RM, Canon F (2010). "Passive stiffness of rat skeletal muscle undernourished during fetal development". Clinics (Sao Paulo). 65 (12): 1363–9. doi:10.1590/s1807-59322010001200022. PMC 3020350. PMID 21340228.
- ↑ "Marasmus and Kwashiorkor". Medscape Reference. May 2009. Archived from the original on 2019-09-04. Retrieved 2022-03-21.
- ↑ Ubesie, Agozie C.; Ibeziako, Ngozi S.; Ndiokwelu, Chika I.; Uzoka, Chinyeaka M.; Nwafor, Chinelo A. (2012-01-01). "Under-five Protein Energy Malnutrition Admitted at the University of In Nigeria Teaching Hospital, Enugu: a 10 year retrospective review". Nutrition Journal. 11: 43. doi:10.1186/1475-2891-11-43. ISSN 1475-2891. PMC 3487930. PMID 22704641.
Further reading
- Bistrian, Bruce R.; McCowen, Karen C.; Chan, Samuel (1999). "Protein–energy malnutrition in dialysis patients". American Journal of Kidney Diseases. 33 (1): 172–5. doi:10.1016/S0272-6386(99)70278-7. PMID 9915286.
- Protein–Energy Undernutrition at Merck Manual of Diagnosis and Therapy Professional Edition
External links
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- Webarchive template wayback links
- Wikipedia articles needing clarification from March 2022
- Articles with invalid date parameter in template
- Articles with hatnote templates targeting a nonexistent page
- All articles with unsourced statements
- Articles with unsourced statements from June 2016
- Protein–energy malnutrition