Vici syndrome
| Vici syndrome | |
|---|---|
| Other names: Dionisi–Vici–Sabetta–Gambarara syndrome | |
Vici syndrome, also called immunodeficiency with cleft lip/palate, cataract, hypopigmentation and absent corpus callosum (or absent corpus callosum cataract immunodeficiency),[1] is a rare autosomal recessive[2] congenital disorder characterized by albinism, agenesis of the corpus callosum, cataracts, cardiomyopathy, severe psychomotor retardation, seizures, immunodeficiency and recurrent severe infections.[3][4] To date, about 50 cases have been reported.[5]
Symptoms and signs
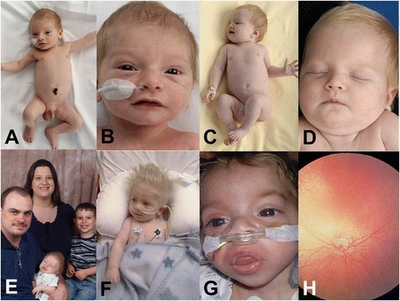
This syndrome consists of a number of typical features. These include[citation needed]
- Agenesis of the corpus callosum (80–99% patients)
- Hypopigmentation of the eyes and hair (80–99% patients)
- Cardiomyopathy (80–99% patients)
- Combined immunodeficiency (80–99% patients)
- Muscular hypotonia (80–99% patients)
- Abnormality of retinal pigmentation (80–99% patients)
- Recurrent chest infections (80–99% patients)
- Abnormal EEG (80–99% patients)
- Intellectual disability (80–99% patients)
- Cataracts (75%)
- Seizures (65%)
- Renal abnormalities (15%)
Infections of the gastrointestinal and urinary tracts are common. Swallowing and feeding difficulties early on may result in a failure to thrive. Optic nerve hypoplasia, nystagmus and photophobia may occur. Facial dysmorphism (cleft lip/palate and micrognathia) and syndactyly may be present. Sensorineural hearing loss may also be present.[citation needed]
Death in infancy is not uncommon and is usually due to cardiac complications or severe infections.[citation needed]
Genetics
Inheritance

Vici syndrome is inherited in an autosomal recessive manner.[2] This means the defective gene responsible for the disorder is located on an autosome, and two copies of the defective gene (one inherited from each parent) are required in order to be born with the disorder. The parents of an individual with an autosomal recessive disorder both carry one copy of the defective gene, but usually do not experience any signs or symptoms of the disorder. The hypothesis of autosomal recessive inheritance of Vici syndrome was strengthened in 2002 with the clinical description of two new cases, one brother and one sister, by Chiyonobu et al.[6]
Gene
Vici syndrome is caused by mutations in the gene EPG5 (OMIM # 615068), which encodes an important regulator of the autophagy pathway, the ectopic P-granules autophagy protein 5, involved in the formation of lysosomes. EPG5 is the human homolog of the C.elegans epg5 gene. The gene EPG5 has been cloned for the first time by Nagase et al. by sequencing clones obtained from a size-fractionated fetal brain cDNA library, and was initially named KIAA1632.[7]
The EPG5 human gene is located on chromosome 18q12.3, has a length of 119,67Kb (NC_000018.10), consists of 44 exons and is transcriptionally driven from the centromere toward the telomere. The messenger RNA (mRNA) is 12633bp long (NM_020964.2) and contains a CDS of 7740 bp translated into a protein sequence of 2579 amino acids (NP_066015.2) with a molecular weight of 280kDa, presumed. The protein EPG5 is expressed primarily in the central nervous system (CNS), skeletal muscle, heart, thymus, cells of the immune system, lungs and kidneys.[8]
Mutations in the EPG5 gene interfere with the autophagy. This appears to be due to a block in the autophagosome-lysosome fusion mechanism.[9]
Diagnosis
The diagnostic workup usually includes an MRI of the brain, an EEG, ophthalmic examination and a cardiac ECHO. Muscle biopsy – which is not commonly done – may show storage of abnormal material and secondary mitochondrial abnormalities in skeletal muscle. Other features that may be seen on muscle biopsy include variability in fibre size, increase in internal and centralized nuclei, type 1 fibre hypotrophy with normally sized type 2 fibres, increased glycogen storage and variable vacuoles on light microscopy
The diagnosis is confirmed by sequencing of the EPG5.
Differential diagnosis
This includes ataxia–telangiectasia, Chédiak–Higashi syndrome, DiGeorge syndrome, Griscelli syndrome and Marinesco–Sjögren syndrome.
Treatment
There is no known curative treatment presently. Hearing aids and cataract surgery may be of use. Control of seizures, heart failure, and treatment of infection is essential. Tube feeding may be needed.
Eponym
Vici syndrome was first described by Carlo Dionisi-Vici et al. (Rome, Italy) in an article from 1988 about two brothers with a previously unreported disorder.[4] Since then, a few articles have reported the same disorder, which subsequently obtained the name Vici syndrome.[10][2]
About 10 years later, del Campo et al. described 4 patients (including 2 sibs, a male and a female) with clinical features very similar to those reported by Dionisi-Vici.[11]
In 2007 the renal tubular acidosis was another clinical complication described in only one case report of two brothers with Vici syndrome.[12]
In 2010 and 2012 it has also been reported a neuromuscular involvement in patients suffering from this syndrome.[13][14]
In 2013 Vici syndrome has been associated with mutations in the gene EPG5 (OMIM # 615068), which encodes an important regulator of the autophagy pathway, the ectopic P-granules autophagy protein 5, involved in the formation of lysosomes.[15]
In 2014 the ophthalmologic features of Vici syndrome were carefully evaluated.[16]
In 2015 the doctoral thesis entitled "Deciphering the mechanism of immune dysfunction in Vici Syndrome", University of Rome "La Sapienza" by Dr. Evangelos Axiotis, clarifies the molecular mechanisms and the role of the mutations in EPG5, all responsible for the immunodeficiency present in patients with Vici syndrome.
References
- ↑ Online Mendelian Inheritance in Man (OMIM): 242840
- ↑ 2.0 2.1 2.2 Chiyonobu T, Y. T.; Yoshihara, T.; Fukushima, Y.; Yamamoto, Y.; Tsunamoto, K.; Nishimura, Y.; Ishida, H.; Toda, T.; Kasubuchi, Y. (April 2002). "Sister and brother with Vici syndrome: Agenesis of the corpus callosum, albinism, and recurrent infections". American Journal of Medical Genetics. 109 (1): 61–66. doi:10.1002/ajmg.10298. PMID 11932994.
- ↑ "Vici syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 18 April 2018. Retrieved 17 April 2018.
- ↑ 4.0 4.1 Vici CD, Sabetta G, Gambarara M, et al. (1988). "Agenesis of the corpus callosum, combined immunodeficiency, bilateral cataract, and hypopigmentation in two brothers". Am. J. Med. Genet. 29 (1): 1–8. doi:10.1002/ajmg.1320290102. PMID 3344762.
- ↑ Byrne S, Dionisi-Vici C, Smith L, Gautel M and Jungbluth H (2016) Vici syndrome: a review. Orphanet Journal of Rare Diseases 11:21 DOI: 10.1186/s13023-016-0399-x
- ↑ Chiyonobu T, Yoshihara T, Fukushima Y, Yamamoto Y, Tsunamoto K et al. (2002) "Sister and brother with Vici syndrome: agenesis of the corpus callosum, albinism, and recurrent infections". American Journal of Medical Genetics 109(1): 61-66.
- ↑ Nagase T, Kikuno R, Nakayama M, Hirosawa M, Ohara O (2000) Prediction of the coding sequences of unidentified human genes. XVIII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res 7(4): 273-281.
- ↑ Cullup T, Kho AL, Dionisi-Vici C, Brandmeier B, Smith F et al. (2013) Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nature genetics 45(1): 83-87
- ↑ Hori I, Otomo T, Nakashima M, Miya F, Negishi Y, Shiraishi H, Nonoda Y, Magara S, Tohyama J, Okamoto N, Kumagai T, Shimoda K, Yukitake Y, Kajikawa D, Morio T, Hattori A, Nakagawa M, Ando N, Nishino I, Kato M, Tsunoda T, Saitsu H, Kanemura Y, Yamasaki M, Kosaki K, Matsumoto N, Yoshimori T, Saitoh S (2017) Defects in autophagosome-lysosome fusion underlie Vici syndrome, a neurodevelopmental disorder with multisystem involvement. Sci Rep 7(1):3552. doi: 10.1038/s41598-017-02840-8
- ↑ del Campo M, Hall BD, Aeby A, et al. (1999). "Albinism and agenesis of the corpus callosum with profound developmental delay: Vici syndrome, evidence for autosomal recessive inheritance". Am. J. Med. Genet. 85 (5): 479–485. doi:10.1002/(SICI)1096-8628(19990827)85:5<479::AID-AJMG9>3.0.CO;2-D. PMID 10405446.
- ↑ del Campo M, Hall BD, Aeby A, Nassogne MC, Verloes A et al. (1999) "Albinism and agenesis of the corpus callosum with profound developmental delay: Vici syndrome, evidence for autosomal recessive inheritance". American Journal of Medical Genetics 85(5): 479-485.
- ↑ Miyata R, Hayashi M, Sato H, Sugawara Y, Yui T et al. (2007) "Sibling cases of Vici syndrome: sleep abnormalities and complications of renal tubular acidosis". Am J Med Genet A 143(2): 189-194.
- ↑ Al-Owain M, Al-Hashem A, Al-Muhaizea M, Humaidan H, Al-Hindi H et al. (2010) Vici syndrome associated with unilateral lung hypoplasia and myopathy. Am J Med Genet A 152A(7): 1849–1853.
- ↑ McClelland V, Cullup T, Bodi I, Ruddy D, Buj-Bello A et al. (2010) Vici syndrome associated with sensorineural hearing loss and evidence of neuromuscular involvement on muscle biopsy. Am J Med Genet A 152A(3): 741-747.
- ↑ Cullup T, Kho AL, Dionisi-Vici C, Brandmeier B, Smith F et al. (2013) "Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy". Nature genetics 45(1): 83-87.
- ↑ J Pediatr Ophthalmol Strabismus. 2014 July 1;51(4):214–20
External links
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- Use dmy dates from June 2019
- Articles with invalid date parameter in template
- All articles with unsourced statements
- Articles with unsourced statements from September 2020
- Congenital disorders
- Autosomal recessive disorders
- Rare diseases
- Syndromes
- Genetic disorders with OMIM but no gene