User:QuackGuru/Sand 35
Can you rewrite the following text 3 different ways? |pmc= https://commons.wikimedia.org/wiki/Special:Watchlist
Adolescent Exposure to Toxic Volatile Organic Chemicals From E-Cigarettes
See "IARC† (No full assessment) • Not generally considered to be a carcinogen • Biological effects through receptor binding • Likely enhances carcinogenicity"[17]
[18] Check for more content. https://nap.nationalacademies.org/download/24625# [1]
See cited by. Connections of nicotine to cancer:[2] 50 sources.


Due to the possibility of dangerous chemicals and flavorings in the aerosol (vapor[21]), there is evidence to suggest that using electronic cigarettes may increase the risk of certain types of cancer (as well as other ailments including cardiovascular and respiratory disease).[19] The presence of carcinogens in the body fluids of e-cigarette users inherently means that cells are at risk of oncogenic transformation.[19] The potential cancer risk associated with e-cigarette use remains a subject of debate.[19] As the most common constituents in e-liquid formulations, propylene glycol and glycerin can produce toxic emissions when heated.[22] The heating procedure of propylene glycol can create plenty of thermal dehydration products such as propylene oxide.[22] As for glycerin, it can generate acrolein and other chemicals.[22]
After using an e-cigarette, nicotine is rapidly metabolized into cotinine and nitrosamines.[22] Nicotine is a potent stimulator of cell proliferation and may stimulate cancer development and growth.[23] The International Agency for Research on Cancer does not consider nicotine to be a carcinogen, though several studies, including studies on pancreatic, breast, and lung cancer, demonstrate it is carcinogenic.[note 1][25] Other cancer-causing substances found in e-cigarette aerosols include formaldehyde, toluene, acetaldehyde, and acrolein as well as heavy metals including cadmium, lead, nickel, nitrosamines, and other substances.[19] Aerosolization of nicotine has proven to generate tobacco-specific nitrosamines, carcinogenic substances, or reactive irritants.[26]
The long-term health effects of using e-cigarettes are not yet fully understood, as the technology is relatively new, and research is ongoing.[19] Nicotine is a highly addictive substance that can have a range of negative health effects, including increased heart rate and blood pressure, constricted blood vessels, and reduced lung function.[19] Bystanders can inadvertently be exposed to the e-cigarette second-hand and third-hand aerosols.[27] Second-hand exhaled exposure to nicotine and cancer-causing chemicals in indoor places may result in serious unwanted effects.[28] Children, particularly young children, may be exposed to the developmental toxicant nicotine from indoor surfaces long after someone had been vaping.[29] Second-hand exposure in indoor environments is of particular concern because people typically spend more than 80% of their time indoors, where emitted pollutants are not diluted as quickly or as extensively as outdoors.[30]
Tobacco smoking is a major cause of preventable premature death worldwide.[31] Tobacco use kills more than eight million people worldwide each year; this number comprises about seven million as a result of direct tobacco use and about 1.2 million passive smokers.[32] Using tobacco can cause not only lung cancer — but also cancers of the mouth and throat, voice box, esophagus, stomach, kidney, pancreas, liver, bladder, cervix, colon and rectum, and a type of leukemia.[33] Smoking can cause cancer almost anywhere in the human body:[34] Tobacco smoke contains approximately 7000 different chemicals, including nicotine, of which 93 chemicals of concern are proposed to produce direct or indirect harm through inhalation, ingestion, or absorption into the body.[23] Heated tobacco products generate both an aerosol[35] and smoke.[36] Prior to 2016, researchers at Philip Morris International stated that their IQOS product produces smoke[36] and the chemical evidence shows that the IQOS emissions fit the definition of both an aerosol and smoke.[37] The emissions of heated tobacco products contain levels of nicotine and carcinogens comparable to classical cigarettes.[38]
Introduction
Composition of e-cigarette aerosol

By heating a liquid that typically has many ingredients, e-cigarettes make an aerosol.[19] Technically, e-cigarettes do not emit vapor.[21] This is because the aerosol has both a particulate and gas phase.[21] E-cigarettes also produce insignificant quantities of incomplete combustion products.[42]
The content of the exhaled aerosol may contain different proportions of harmful constituents depending upon the user's technique or other factors, such as temperature, weather, and airflow.[43] Individuals breathe this aerosol into their lungs, when a user exhales into the air, this aerosol could also be inhaled by non-users.[19] Vapers produce an aerosol made up of a mixture of liquid droplets.[note 3][19] It has been indicated that the aerosol size in the indoor environment is less than 50 nm.[19] As a result of the enormous surface area of the alveolar region's airways (about 75 m2[44]), a substantial amount of the breathed in e-cigarette aerosol is thought to penetrate and deposit deep within the lungs.[19]
E-cigarette liquid
E-liquids are the liquid solutions that are used in e-cigarettes to produce aerosol.[19] It typically consists of propylene glycol and glycerin, flavors, nicotine, formaldehyde, and other chemicals that are heated, aerosolized, and inhaled.[19] E-cigarettes can be used to aerosolize cannabis-infused concentrates.[45] These devices can also be used to aerosolize tetrahydrocannabinol (THC) or cannabidiol.[45] The current evidence indicates that e-liquids often contain a variety of potentially toxic chemicals.[19] There are also chemicals unique to flavored e-liquids that are not found in classical cigarettes, such as propylene glycol, glycerin, and various flavoring agents.[46]
Propylene glycol and glycerin are both used as carriers for the flavorings and nicotine in e-liquids.[19] Propylene glycol is a thinner liquid that produces a stronger throat hit and a more intense flavor, while glycerin is a thicker liquid that produces more aerosol and a sweeter taste.[19] The ratio of propylene glycol to glycerin in an e-liquid can affect the overall flavor, throat hit, and aerosol production.[19] For example, a higher percentage of propylene glycol seems to enhance flavor and strengthen the throat hit, whereas a higher percentage of glycerin may increase aerosol production.[note 4][48]
Flavorings are added to e-liquids to provide a wide range of tastes and aromas.[19] Some common flavors include fruit, candy, dessert, and menthol.[19] Mint flavors contain menthol or menthone and candy flavors may contain vanillin and cinnamaldehyde.[23] E-liquids can contain nicotine, which is an addictive substance found in tobacco.[19] The concentration of nicotine in an e-liquid can vary, and users can choose from a range of strengths to suit their preferences.[19] Not only is there evidence of mislabeling of nicotine content among refills labelled as nicotine-free, but there also seems to be a history of poor labelling accuracy in nicotine-containing e-liquids.[3]
Background

E-cigarettes do cause the inhalation of carcinogenic substances.[19] Due to the possibility of dangerous chemicals and flavorings in the aerosol, there is evidence to suggest that using e-cigarettes may raise the risk of certain types of cancer (as well as other ailments including cardiovascular and respiratory disease).[19] The presence of carcinogens in the body fluids of e-cigarette users inherently means that cells are at risk of oncogenic transformation.[19] The potential cancer risk associated with e-cigarette use remains a subject of debate.[19] E-cigarettes work by heating a liquid that usually contains nicotine, flavorings, and other chemicals.[19] When the liquid is heated, users inhale an aerosol into their lungs.[19] E-cigarettes contain potentially harmful chemicals, which can damage DNA and lead to cancer.[19]
Several studies have investigated the potential cancer risk associated with e-cigarette use, while other studies have suggested that e-cigarette aerosol may contain carcinogenic chemicals that could increase the risk of lung and bladder cancer in humans.[19] However, according to a 2023 review, these studies are limited in their scope and do not provide conclusive evidence.[19] Overall, the long-term cancer risk associated with e-cigarette use remains uncertain.[19]
The high temperatures (above 200 °C) that are achieved by e-cigarette solutions produce tobacco-specific nitrosamine compounds, acetaldehyde, a possible carcinogen, metals, nitrosamines, and carbonyl compounds including acrolein and formaldehyde, which are human carcinogens, according to the International Agency for Research on Cancer.[19] Several of the same toxicants found in classical cigarettes such as acetone, acetaldehyde, and formaldehyde are also found in e-cigarette aerosols.[52] When the e-cigarette aerosol is inhaled it accumulates within the respiratory epithelium in a manner similar to smoke from classical cigarettes.[52] Although the quantities produced by e-cigarettes are lower than those in tobacco smoke, they are nevertheless adequate to contribute to carcinogenesis because they contain the recognized carcinogens formaldehyde and acrolein.[19] E-liquids without nicotine can produce high levels of carbonyl compounds,[26] and there is strong evidence that e-liquids without nicotine contains potentially cancer-causing chemicals.[53]
As the most common constituents in e-liquid formulations, propylene glycol and glycerin can produce toxic emissions when heated.[22] The heating procedure of propylene glycol can create plenty of thermal dehydration products, mainly including acetaldehyde, formaldehyde, propylene oxide, acetol, allyl alcohol, glyoxal, and methylglyoxal.[22] As for glycerin, it can generate acrolein and formaldehyde, as well as dehydrated glycerin.[22] There is also a variety of unidentified chemicals in the e-cigarette aerosol.[54]
For years, the tobacco industry has used deceptive marketing and advertising practices to target specific groups, such as young individuals and minorities.[55] A main tactic used by the tobacco industry involves introducing new products that are promoted as safe options to traditional tobacco products.[56] E-cigarettes, and other nicotine-enriched products, are being vigorously marketed as "magical remedies"[25] and the leading promotors of vaping products are large tobacco companies.[57] The surge in e-cigarette use among young people seems to align with the intense and possibly deliberate younger audience-targeted advertising efforts of certain e-cigarette companies like Juul Labs.[53] Some young individuals who have never smoked have tried using e-cigarettes.[58] By promoting e-cigarettes as a healthier option, tobacco companies are aiming to rebrand themselves.[59] The marketing claim that e-cigarettes are 95% less dangerous than classical cigarettes has not been substantiated.[note 5][61] E-cigarettes are frequently viewed as a safer alternative to conventional cigarettes; however, evidence to support this perspective has not materialized.[20] Research has shown that the aerosolization process can lead to the creation of harmful substances, including formaldehyde, even when they were not initially present in the e-liquid solutions.[62] Consequently, the predisposition that the aerosolization process is a safe substitute for combustion has been called into question.[62] As the tobacco epidemic has waned, it has been followed by the entrance of e-cigarettes primarily being offered by the tobacco industry to try to recruit replacements for the deceased tobacco users.[note 6][63]
Information on various e-cigarettes and e-cigarette liquids


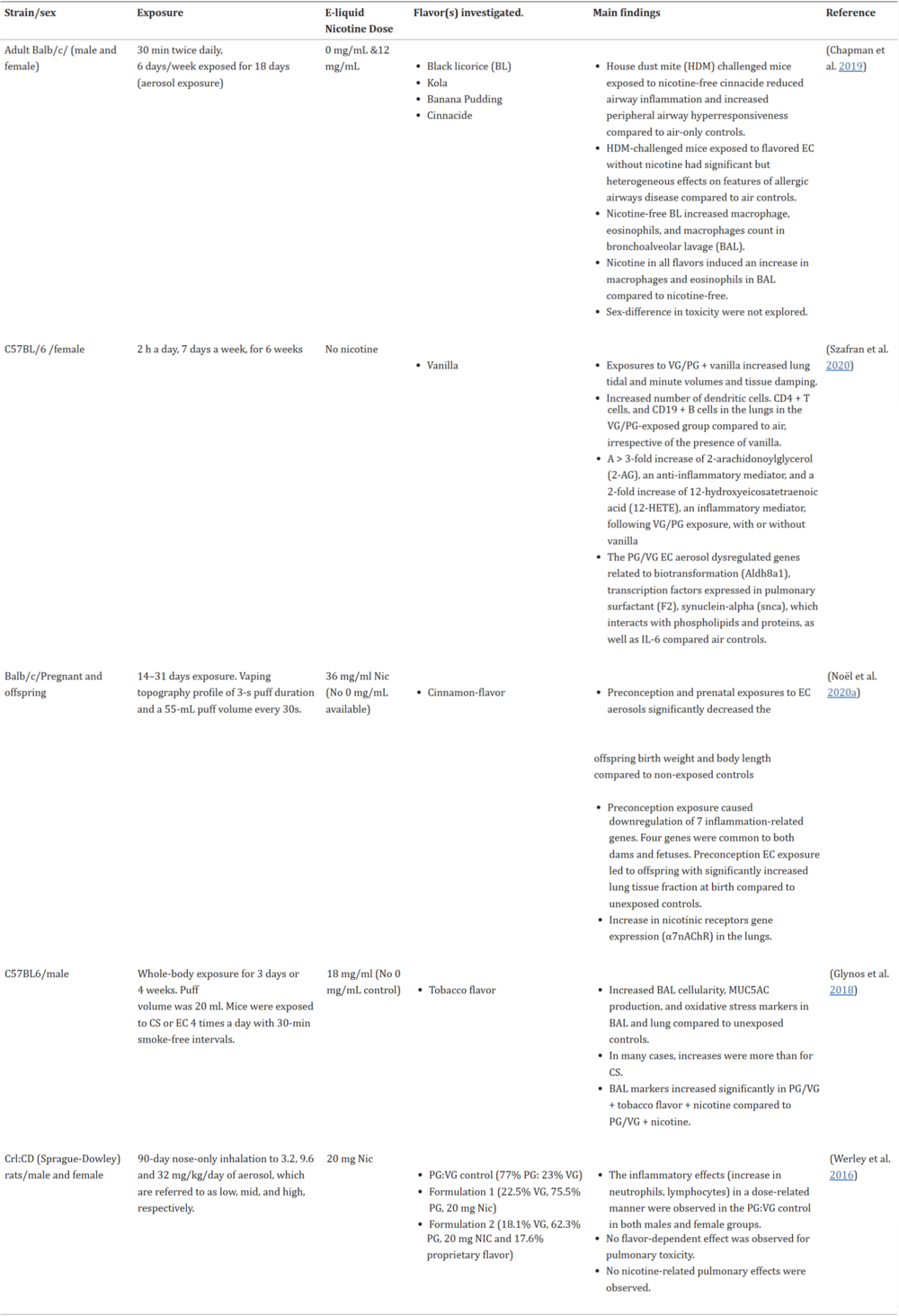
Information on mean concentrations of carcinogenic compounds contained in aerosol of e-cigarettes versus smoke of traditional cigarettes

Information on urine levels of metabolites of hazardous compounds in e-cigarette-only users versus dual users and non-smokers

Causative agents
Acetaldehyde
Acetaldehyde has been classified by the National Academy of Medicine as the most important cardiovascular toxicant in tobacco smoke.[19] In addition to tobacco smoke, acetaldehyde has also been found in cigars and hookahs (water pipes), and e-cigarette aerosols.[19] The risk of cancer from acetaldehyde exposure may be particularly significant for individuals who use e-cigarettes over the long term, as repeated exposure to the chemical can lead to the accumulation of DNA damage and other cellular changes that increase the risk of cancer.[19] The International Agency for Research on Cancer has categorized acetaldehyde as a possible carcinogen to humans (group 2B).[65]
Studies have shown that the levels of acetaldehyde in e-cigarette aerosols can vary widely depending on the type of e-cigarette device, the power setting, and other factors.[19] However, even at low levels, acetaldehyde has been shown to have carcinogenic properties and has been linked to an increased risk of cancer, particularly in the upper respiratory tract and the head and neck area.[19] Acetaldehyde is a carcinogen and may promote cancer development through multiple mechanisms, including interfering with DNA replication, inducing DNA damage, and forming DNA adducts.[19]
Acrolein
Acrolein has been classified by the National Academy of Medicine as the most important cardiovascular toxicant in tobacco smoke.[19] Acrolein is a toxic chemical that is present in both tobacco smoke and e-cigarette aerosol.[19] It is formed when glycerin, a common ingredient in e-liquids, is heated during the vaping process.[19] Acrolein is a known respiratory irritant and can damage DNA, which can lead to cancer.[19] Several studies have investigated the potential cancer risk associated with acrolein exposure from e-cigarette use.[19] The International Agency for Research on Cancer has classified acrolein as probably carcinogenic (group 2A) to humans.[66]
Some studies have suggested that e-cigarette aerosol may contain levels of acrolein that are higher than those found in tobacco smoke.[19] One study found that acrolein levels in e-cigarette aerosol were up to 14 times higher than those found in tobacco smoke.[19] Acrolein was also found to form DNA adducts in p53 mutational hotspots similar to those found in smoking-related lung cancers, suggesting that acrolein may be a relevant etiologic agent involved in e-cigarette use.[19] Numerous studies have demonstrated that chronic exposure to acrolein promotes cardiovascular disease, whereas even low-level acute exposure to the substance causes dyslipidemia, vascular damage, endothelial dysfunction, and platelet activation.[19] Acrolein is involved in the development of cancer, according to investigations on animals.[19]
Cannabis and its derivatives
Epidemiological studies examining the link between cannabis use and various cancers have resulted in different conclusions.[67] Research on cannabis on the risk of causing lung cancer (and other effects) has been historically limited due to its former illegal classification and the intertwined effects of habitual tobacco use.[68] Cannabis consumption is correlated with the development of cancers of the head and neck, larynx, lung, leukemia, brain, prostate, cervix, testicles, and bladder.[69] Cannabis use greatly harms DNA, which leads to a higher occurrence of zygote death.[69] Depending on the dose and length of use, cannabis can also cause cancer and genetic changes.[70] The amount of time needed to cause an increased risk of cancer from cannabis use is uncertain.[71]
The 2017 The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research book states, "There is insufficient evidence to support or refute a statistical association between parental cannabis use and a subsequent risk of developing acute myeloid leukemia/acute non-lymphoblastic leukemia, acute lymphoblastic leukemia, rhabdomyosarcoma, astrocytoma, or neuroblastoma in offspring."[72] Reports have linked cannabis use to the growth of tumors, including in children whose mothers' used cannabis during pregnancy.[70] In children whose mothers used cannabis before or during gestation, it causes a 10-fold increase in the risk of non-lymphoblastic leukemia and it can increase the risk of chromosomal damage (including breakage and translocation), damaging mainly the somatic cells.[73]
Elevated temperatures are needed to aerosolize the THC present in cannabinoid oil.[74] Consequently, vaping devices may need to operate at a stronger power compared to those used for traditional e-liquids in order to get the preferred effect.[74] At these increased temperatures, the e-liquid ingredients may undergo pyrolysis, which may lead to the formation of dangerous or carcinogenic chemicals.[74]
Cannabis use might carry a risk of cancers of the head, neck, and pharyngeal areas.[71] There appears to be a relationship between cannabis use and bladder cancer.[71] The current research suggests there is a relationship between cannabis use and testicular germ cell tumors.[71]
Cannabis e-liquids are prone to thermal decomposition and pyrolysis, which yield a diverse potential of toxic organic compounds.[75] Cannabidiol vaping products have been shown to oxidize into a reactive cannabinoid quinone, which generates adducts with protein cysteine residues, which leads to altered protein function.[75] Cannabinoid quinone was found to induce cytotoxicity, apoptosis in specific cells, liver toxicity, and inhibit topoisomerase II and angiogenesis.[75] It has been shown that aerosolized cannabidiol induces apoptosis, pro-inflammatory reactions, reactive oxygen species generation, and enhanced cytotoxicity in bronchial epithelial cell lines.[75] The potential for cannabis oncogenicity could be attributed to toxic and pro-inflammatory effects on respiratory functions.[75] Cannabis creates molecular histologic alterations to the bronchial epithelium which resembles that caused by using tobacco products.[68]
The biological plausibility of the link between cannabis exposure and testicular cancer is thought to be related to disruptions to the hypothalamic–pituitary–testicular axis – an endocrine feedback system which, among other actions, assists with spermatogenesis.[76] It is thought that cannabis exposure – and subsequent stimulation of cannabinoid receptors – disrupts normal hormone regulation and testicular function, and that this disruption leads to carcinogenesis.[76] However, evidence regarding the association between regulation of normal testicular function and tumor development remains inconclusive; and given the complex and multifaceted influence of cannabinoid receptor stimulation on biological processes, the path from cannabis exposure to testicular carcinogenesis remains unclear.[76]
Current, chronic, and frequent cannabis use is associated with the development of testicular tumor germ cell tumors – particularly non-seminoma testicular germ cell tumors – at least when compared to never-users of cannabis.[76] The strongest association was found for non-seminoma development--for example, those using cannabis on at least a weekly basis had two and a half times greater odds of developing a non-seminoma testicular germ cell tumor compared those who never used cannabis.[76] There is inconclusive evidence regarding the relationship between ever- and former-use of cannabis and testicular germ cell tumor development.[76] A 2015 review noted that these observations were derived from only three published studies; that these studies were all conducted in the US; and the majority of data collection occurred during the 1990’s.[76]
Diethylene glycol
In 2014, the US Food and Drug Administration identified around 1% of diethylene glycol in one of the 18 e-cigarette cartridges analyzed.[77] The contamination was thought to arise from poor quality propylene glycol.[77] Diethylene glycol, an ingredient used in antifreeze, is toxic to humans.[78]
Ethanol
Ethanol is classified as a carcinogen (group 1[79]) by the International Agency for Research on Cancer.[19] This is the most severe classification.[79] Although there are rules for disclosing substances, including ethanol, in e-liquids in other nations, ethanol has been noted as an undeclared ingredient in nicotine-containing e-liquids sold in the US.[19] Ethanol alters epigenetics by altering DNA and histone methylation and acetylation.[19] This may affect the regulation of gene expression even after transplacental exposure.[19] On the other hand, the evidence for the carcinogenicity of ethanol in laboratory animals is insufficient.[19] However, no concrete evidence has been found that ethanol in e-liquids can cause cancer.[19]
Ethylene glycol
The majority of e-liquids are absent of containing ethylene glycol.[80] A 2014 study showed that e-liquids from a specific manufacturer contained greater amounts of ethylene glycol than glycerin or propylene glycol, but ethylene glycol has not been permitted for use in products meant for human consumption.[81] A 2018 study found measurable levels of the metabolites of ethylene glycol in e-cigarette users.[62] In comparison to traditionally utilized glycerin and propylene glycol, ethylene glycol acts as an irritant to the respiratory system and it is linked to considerably greater toxicological risks.[80]
Ethyl maltol
Foods frequently include ethyl maltol, a flavoring ingredient that is regarded as generally harmless.[19] The aerosols of numerous commercial e-cigarette devices have been found to contain ethyl maltol.[19] Research has been conducted to determine whether ethyl maltol increases heavy metal-mediated toxicity because ethyl maltol accelerates heavy metal transport across plasma membranes and heavy metals have been found in aerosols produced by e-cigarettes.[19]
Further radical generation has been found to come from ethyl maltol's interaction with iron and copper, which are typically present in the heating element and/or as impurities.[19] Additionally, it has been shown to promote additional pro-inflammatory effects and enhance systemic exposure to inhaled chemicals, as well as to trigger an inflammatory response, modify local immune function, and damage epithelial barrier function and integrity.[19] This strongly shows that ethyl maltol is carcinogenic given the proven oncogenicity of free radicals both individually and collectively.[19]
Flavoring
The flavoring chemicals added to e-liquids adds an extra layer of complexity to the toxicity of e-cigarettes.[22] The mode of toxicological actions for many of the flavors in e-liquids remains elusive.[23]
A 2021 review identified 65 unique flavor substances in e-cigarette aerosols that stimulate toxicity in the circulatory system, skeletal system, respiratory tract, and skin.[82] The most commonly cited cytotoxics were cinnamaldehyde, vanillin, menthol, ethyl maltol, ethyl vanillin, benzaldehyde, and linalool.[82] Data collected demonstrated greater detrimental effects in vitro with cinnamon, strawberry, and menthol, flavors than other flavors.[23] The most reported effects among these investigations were perturbations of pro-inflammatory biomarkers and enhanced cytotoxicity.[23] The evidence suggests that flavors such as cinnamon, menthol, strawberry, tobacco, and many others, induce one or more of the following adverse effects: mitochondrial dysfunction, cell death, reactive oxygen species production, and dysregulation of inflammatory cytokines.[23]
Flavoring agents in e-cigarettes, including vanillin, ethyl vanillin, and benzaldehyde, can react with the e-liquid solvent, propylene glycol, to form acetals that can efficiently transfer to e-cigarette aerosol, and as a result may be more toxic compared with their parent aldehydes.[49]
Formaldehyde
The International Agency for Research on Cancer has categorized formaldehyde as a human carcinogen (group 1).[65] Formaldehyde-containing hemiacetals were observed to be detectable by nuclear magnetic resonance spectroscopy during the aerosolization process.[19] Propylene glycol and glycerin are known formaldehyde releasers.[19] Formaldehyde-releasing compounds averaged 38,090 g per sample (10 puffs) at high voltage (5.0 V), according to an analysis of a commercial e-liquid used in a "tank system" e-cigarette with a variable voltage battery.[19] It might accumulate in the respiratory system more quickly than gaseous formaldehyde, which might increase the likelihood of developing cancer.[19] This risk is five times greater than the risk of chronic smoking.[19]
Glycerin
Limited animal or human data exists involving the possible toxic effects of breathing in glycerin.[83] According to toxicological research, glycerin is associated with less irritation to the upper respiratory tract than propylene glycol.[84] In an in vitro 2018 study, aerosols from glycerin only-containing refills showed cytotoxicity in A549 cells and human embryonic stem cells, even at a low battery output voltage.[3] The effect of heating glycerin is carcinogenic.[85]
Menthol
Modulation of nicotine metabolism and direct carcinogenic/pro-inflammatory effects are the two main mechanisms by which menthol exerts its potentially cancer-causing effects.[19] Menthol is also linked to nicotine, both indirectly (via direct effects on endogenous responses to nicotine, such as through modulation of nicotinic receptor expression) and directly (through greater tolerance/reduced throat irritation of tobacco smoke and e-cigarettes.[19] It is linked to an overall rise in the prevalence of addiction.[19] When exposed to various aromatic compounds, including those with menthol as an ingredient, lung cancer cells' ability to invade and metastasize was demonstrated to increase, according to a 2018 study.[19]
Metals

Generally, metals/metalloids exist in soldered joints and coils, which are made of alloys or high-purity metals.[22] Studies have found most metal/metalloid bio-sample levels in e-cigarette users were similar or even higher in comparison with tobacco cigarette users and higher in comparison with cigar users.[22]
According to certain research, dangerous heavy metals like cadmium and lead can be discovered in both cigarette smoke and the aerosol produced by e-cigarettes.[19] Toxic heavy metals like lead, nickel, chromium, and manganese can be present in higher concentrations in e-cigarette aerosols and e-liquids than in cigarettes, according to a study by the California Department of Public Health.[19] Part of the heavy metals present in the e-cigarette aerosol, such as lead, are derived from Nicotiana tabacum, the source of nicotine in tobacco, which absorbs pollutants from the environment during its growth.[32] E-cigarette emissions may contain arsenic[86] and it is classified as carcinogenic to humans (group 1) by the International Agency for Research on Cancer.[87] In humans, arsenic causes skin, lung, bladder, prostate, kidney, and liver cancers.[87]
Metal nanoparticles generated from the heating coil components have also been detected in the e-cigarette aerosols.[88] As opposed to larger particles, nanoparticles possess an enlarged surface area relative to their mass, which amplifies their ability to act as catalysts.[7] Due to their miniscule size, nanoparticles can penetrate and travel more easily across cellular barriers which enables them to reach different parts of the body, such as the brain.[7] Titanium dioxide nanoparticles that have been found in the e-cigarette aerosols can interfere with DNA repair processes.[89] This can happen as a result of single-strand breaks and oxidative damage to the DNA within the A549 cells.[89] In comparison to cigarette smoke, e-cigarette aerosol generally produces high levels of nanoparticles and less larger particles (below 10 μM).[7]
A 2013 study found that the concentrations of nickel in e-cigarette aerosol were 100-fold greater than in classical cigarettes.[21] A 2018 study found significantly higher amounts of metals in e-cigarette aerosol samples in comparison with the e-liquids before they came in contact with the customized e-cigarettes.[90] For example, nickel and tin were 600% higher in the e-cigarette aerosol than in the e-liquid.[90] In comparison to classical cigarette users in a 2018 study, e-cigarette users were found to have greater serum concentrations of certain rare-earth elements such as selenium, silver, vanadium, and lanthanides.[7] Mixtures of various metals and other substances, even when present at amounts deemed beneath the individual lowest-observed-adverse-effect level, can result in cumulative or synergistic effects.[7]

Heavy metal exposure, which is caused by e-cigarettes, can have detrimental consequences on health.[19] Respiratory conditions like lung cancer have been linked to nickel and chromium from industrial exposure, and these substances have been found in the aerosols of some e-cigarette brands.[19] Moreover, in e-cigarettes, heating coils are usually made of nichrome, which is a combination of nickel and chromium and stainless steel.[92] Nickel and chromium are classified as carcinogenic to humans (group 1) by the International Agency for Research on Cancer.[92] Nickel (as well as ethylene oxide) has been linked to lung and sinus cancers, lymphomas, multiple myeloma, and leukemia.[66]
Neurological and developmental issues can result from lead and manganese exposure.[19] Cadmium exposure is linked to lung cancer and can harm the kidneys.[19] Generally, small amounts of contaminates from the e-cigarette device itself may include metals from the heating coils, solders, and wick.[93] The metal contaminants may include lead, cadmium, nickel, chromium, silver, tin, and silicates.[93] Parts of the e-cigarette, such as exposed wires, wire coatings, solder joints, electrical connectors, heating element material, and vitreous fiber wick material, may be inhaled by the e-cigarette user.[94] Following e-cigarette use, thermal decomposition of certain substances and possible breakage of wick fibers due to heat can occur near the heating element.[94] The components in e-cigarettes that raise the risk of cancer are not just heavy metals.[19] In addition to being carcinogens, other compounds including formaldehyde and acetaldehyde also present extra risks to e-cigarette users.[19] In summary, e-cigarettes have a lot of components that could make users more likely to get cancer.[19]
Nicotine
—Aseem Mishra and colleagues, Indian Journal of Medical and Paediatric Oncology[25]
Compared to other poisons, nicotine is one of the more toxic poisons, and it negatively impacts several organs of the human body.[25] For young individuals, e-cigarette use itself has no identifiable benefit, but there are serious concerns regarding their effects.[95] Nicotine is particularly potent in children and young adults.[96] Nicotine itself has not been proven to have any meaningful favorable effect.[25] Newer vaping products can enhance the amount of aerosol generated and can increase the bioavailability of nicotine.[97]
Nicotine exhibits a range of diverse and intricate actions.[98] Nicotine inherently possesses properties that promotes the growth of tumors.[99] Nicotine itself poses numerous health hazards by influencing several processes, such as cell proliferation, apoptosis, and the immune response; however, it also contributes to oxidative stress and subsequent DNA mutations, which can lead to cancer.[32] Nicotine exposure is associated with an increased probability of tumor proliferation, metastasis, and can also increase the likelihood of treatment failure by reducing the effectiveness of chemotherapy and radiotherapy.[32] It possesses the ability to permeate any human membrane.[100] This includes the brain and placenta.[46]

Although the International Agency for Research on Cancer does not consider nicotine to be a carcinogen, several studies, including studies on gastrointestinal, breast, and lung cancer, demonstrate it is carcinogenic.[note 7][25] Nicotine is reportedly directly associated with causing the following cancers: small-cell and non-small-cell lung carcinomas, in addition to head and neck, gastric, pancreatic, gallbladder, liver, colon, breast, cervical, bladder, and kidney cancers.[103] The evidence suggests that nicotine may promote cancer progression in an independent manner that is separate from the effects of the combustion products of tobacco smoke.[102] Because it can form nitrosamine compounds (particularly N-Nitrosonornicotine (NNN) and nicotine-derived nitrosamine ketone (NNK)) through a conversion process, nicotine itself exhibits a strong potential for causing cancer.[53] About 10% of breathed in nicotine is estimated to convert to these nitrosamine compounds.[53]
Nitrosamine carcinogenicity is thought to be a result of enhanced DNA methylation and may lead to an agonist response on the nicotinic acetylcholine receptors, which acts to encourage tumors to grow, stay alive, and penetrate into neighboring tissues.[53] Although nicotine in the form of nicotine replacement products is less of a cancer risk than with smoking,[104] there is evidence that nicotine may cause oral, esophageal, or pancreatic cancer.[105] Nicotine has a strong tumor-inducing effect on several kinds of cancers.[52] This is because nicotinic acetylcholine receptors are present on the surfaces of both tumor and immune cells, which allows nicotine to directly impact the surrounding environment of the tumor.[52]
Prolonged exposure to nicotine or its carcinogenic by-products increases the activity of nicotinic acetylcholine receptors that encourage cancer growth and diminishes the effectiveness of nicotinic acetylcholine receptors that inhibit cancer.[106] Nicotine appears to be a strong mitogenic agent.[107] This is because it stimulates cell proliferation in tumors.[107] By virtue of its tumor-promoting effects, nicotine works together with other carcinogens such as car exhaust and may decrease the time of cancers to initiate.[25]
The limited evidence suggests that refillable e-liquids may contain impurities and nicotine breakdown by-products, including nicotine-cis-N-oxide, nicotine-trans-N-oxide, anabasine, anatabine, and myosmine.[108] These chemicals are highly carcinogenic and may alter genes that are vital for controlling cell growth and suppressing tumors, such as Ras, p53, and retinoblastoma.[108] This is because of their ability to attach to cellular DNA, creating adducts that can result in genetic mutations.[108]
Metastasis is a major contributor to cancer deaths, and the epithelial-to-mesenchymal transition serves as a key indicator of metastasis.[109] Exposure to e-liquid and e-cigarette aerosol led to a substantial increase in the indicators of the epithelial-to-mesenchymal transition in the adenocarcinoma alveolar basal epithelial cells.[109] This exposure also results in the cells converting to a fibroblast-like shape, the breakdown of cell-to-cell junctions, internal repositioning of E-cadherin, a rise in motility, and the relocation of active β-catenin to the cell nucleus.[109]
A 2023 review concluded that "There is growing evidence through the use of animal xenograft models and cell culture systems, that (1) nicotine's carcinogenic role stems from multiple signaling mechanisms, primarily involving both non-receptor-mediated actions and receptor-mediated effects, including nicotinic acetylcholine receptors, β-adrenergic receptors and epidermal growth factor receptors, as well as transforming growth factor β receptors; (2) nicotine could induce chromosomal abnormalities, DNA damage, and micronuclei formation; (3) nicotine also can enhance oxidative stress, leading to tumor initiation or progression due to excessive production of reactive oxygen species. Based on these findings, nicotine seems to be a potent oncogenic agent in modulating tumor cell proliferation, invasion and migration by various signaling pathways associated with chemical carcinogenicity."[24]
Prenatal and neonatal exposure to nicotine
Nicotine exposure during pregnancy and during infancy, whether through nicotine replacement products or cigarette smoking, could elevate the risk of cancer later in life.[110]
Nicotine-free e-cigarette aerosol
Nicotine-free e-cigarette aerosols still contains chemicals that have been linked to cancer.[111] Although they do not produce cigarette smoke and thus do not contain the subsequent by-products such as tar, ash, and carbon monoxide,[note 8][113] e-cigarette aerosols do contain many of the same toxic chemicals and carcinogens that are found in cigarette smoke.[55] Research indicates that formaldehyde, acetaldehyde, and reactive oxygen species are at high enough levels to inflict inflammatory harm to the cells lining the airways and lungs.[68]
A 2016 study on head and neck squamous cancer and healthy epithelial cell lines subjected to e-cigarette aerosols from various brands, even without nicotine, led to a decrease in cell survival rates and a noticeable increase in cell death and tissue decay, in contrast to the unexposed control group.[109] Moreover, the exposed cell lines demonstrated a greater expression of H2A histone family member X (γ-H2AX), which is a recognized indicator for double-strand breaks in DNA.[109] Irrespective of the nicotine content, e-cigarette aerosol has been identified as cytotoxic and an agent that can break DNA strands.[109]
The evidence suggests that even nicotine-free e-cigarette aerosols may cause harm to the fetus.[114] The HTR8/SVneo cells derived from transfected cells of human chorionic villi have been used to study the function of placental cells exposed to flavorless e-cigarette without nicotine, showing a significant reduction in trophoblast impairment and angiogenesis functions, which are vital for placental circulation.[114] These results suggest that placental cells may be vulnerable to exposure to EC aerosols, even in the absence of nicotine.[114]
Propylene oxide
When propylene glycol is heated and aerosolized, it could turn into propylene oxide.[115] The International Agency for Research on Cancer states it is a possible carcinogen (group 2B) to humans.[115]
A 2018 study detected significantly higher levels of metabolites of hazardous compounds in the urine of adolescent dual users (e-cigarettes and conventional tobacco consumers) than in adolescent e-cigarette-only users.[3] Moreover, the same study observed that the urine levels of metabolites of propylene oxide, as well as acrolein, acrylamide, acrylonitrile, and crotonaldehyde, all of which are detrimental for human health, were significantly higher in e-cigarette-only users than in non-smoker controls, reaching up to twice the registered values of those from non-smoker subjects.[3]
Tobacco-specific nitrosamines
In order to create a variety of alkaloid compounds, tobacco plants undergo biochemical processes.[117] The alkaloids in tobacco include nicotine, (3‐(1‐methyl‐2‐pyrrolidinyl) pyridine, nornicotine, anabasine, anatabine, and myosmine.[118] The noxious alkaloids that are in Nicotiana plants mainly act as defense compounds to fend off generalist herbivores.[119] The amount of nicotine in tobacco leaves is influenced by various factors such as cultivation practices, environmental conditions, and genetic background.[120] In most tobacco varieties, nicotine is the predominant alkaloid, typically comprising over 90% of the total alkaloid pool.[120] While the dominant form of nicotine found in tobacco is S-nicotine, the R-stereoisomer (R-nicotine) is also found at detectable levels.[121]
Tobacco-specific nitrosamines are compounds that are formed from tobacco alkaloids.[122] They are exclusively found in tobacco products.[118] They are formed through the nitrosation of tobacco alkaloids during the tobacco curing and fermentation process.[97] The tobacco-specific nitrosamines NNN and NNK are mainly formed through the nitrosation process of their precursor amines, pseudooxy nicotine and nornicotine, which are found in tobacco.[123] Pseudooxy nicotine is present in tobacco in both free and matrix-bound forms.[97] They can also be formed by the nitrosation of nicotine,[123] which involves the reaction of nicotine with nitronium ions.[42] Substandard storage conditions and production methods of e-liquid and tobacco flavorings have been associated with the generation of nitrosamines and an increase in their amounts.[109]
NNN and NNK are among the most carcinogenic tobacco-specific nitrosamines, according to animal research.[122] In a considerable number of cases, there is a strong correlation between the creation of DNA adducts and the carcinogenic effects of tobacco-specific nitrosamines, according to animal research.[122] This association is assumed to be similar in humans.[122] Tobacco-specific nitrosamines can induce cancer initiation in multiple human organs, including the esophagus, pancreas, lung, liver, and bladder.[100]
Tobacco-specific nitrosamines have been found in e-cigarette refillable liquids, cartridges, and aerosols.[88] Exposure to all tobacco-specific nitrosamines was lower among people who vaped compared to people who smoked.[97] Levels were higher among people who vaped compared to people who neither vaped nor smoked.[97] The urinary concentrations of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) was reportedly lower in smokers who migrated to e-cigarettes.[94] NNN has also been detected in e-cigarette users, with median values of 2.15 pg/mL and 0.1 fmol/mL in the saliva and urine.[123]
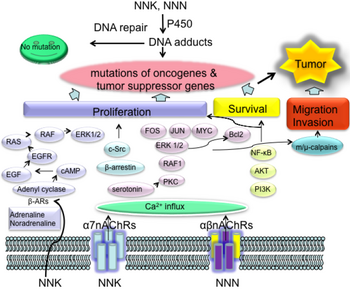
NNK and NNN oncogenic metabolites may induce the formation of DNA adducts that leads to mutations of tumor suppressor genes including Rb and p53.[124] Nicotine or NNK signaling may contribute to cancer progression.[124] Nicotine was implicated in promoting the self-renewal of stem-like side-population cells from lung cancers.[124] The subpopulation of cancer stem-like cells was implicated in tumor initiation, generation of heterogeneous tumor populations, metastasis, dormancy, and drug resistance.[124] Furthermore, nicotine can inhibit apoptosis induced by opioids, etoposide, cisplatin, and UV irradiation in lung cancer cells.[124] Therefore, the activation of nicotine signaling might be associated with drug resistance in lung cancer.[124] The nicotine-derived metabolites including NNK and NNN are potent carcinogens because they bind to α7nAChR.[124] The binding activity of NNK to α7nAChR was 1,300 times greater than that of nicotine.[124] Nicotine signaling triggers the production of β-AR ligands, such as adrenaline and noradrenaline, which contribute to the development of lung cancer.[124]
NNN and NNK may be formed from nicotine after oral administration.[102] After uptake of nitrosamines, including but not limited to NNK and NNN, they are metabolized by cytochrome P450s, and the resulting metabolites ultimately breakdown into formaldehyde, methyldiazohydroxides, and pyridylic-butylic by-products.[42] Although all of these by-products can potentially harm DNA, the cancer-causing characteristics of nitrosamines are primarily associated with methyldiazohydroxides.[42] Methyldiazohydroxides can provoke the formation of mutagenic and carcinogenic O6-methyl-deoxyguanosines adducts, and can also form minuscule adducts of methylated thymine and methylated cytosine.[42] NNN and NNK are classified by the International Agency for Research on Cancer as human carcinogens (group 1[125]).[102]
NNK and NNN have been associated with lung, liver, esophageal, and pancreatic cancers in animal studies.[97] NNK has also been reported to have a dose-dependent effect on the risk of lung cancer in humans.[97] Animal studies have shown that NNN specifically causes esophageal and nasal tumors in rats and respiratory tract tumors in mice and hamsters.[116] Three types of reactions have been observed in NNN metabolism pathways: pyridine N-oxidation, hydroxylation of the pyrrolidine ring (including α-hydroxylation at the 2'- and 5'-positions and β-hydroxylation at the 3'- and 4'-positions), and norcotinine formation.[116] The 2'- and 5'-α-hydroxylation pathways are the major pathways leading to the formation of DNA adducts.[116] 2'-Hydroxy NNN undergoes spontaneous ring opening to produce a pyridyloxobutyldiazohydroxide identical in structure to that formed upon methyl hydroxylation of NNK.[116] 5'-Hydroxylation also yields an electrophilic diazohydroxide, which is expected to react with DNA, and the α-hydroxylation reactions of NNN are catalyzed predominantly by CYPs.[116]
Although DNA adduct formation is considered the central step in the process of NNK and NNN carcinogenesis, the capacity of various DNA adducts to induce mutations and chromosomal aberrations varies extensively.[116] O6-mGua is a highly pro-mutagenic adduct causing G:C to A:T transitions.[116] O6-mGua adducts can be removed by the DNA repair protein, O6-alkylguanine DNA-alkyltransferase (AGT; also known as MGMT) or AlkB homologs.[116] AGT overexpression in transgenic mice reduces the formation of K-ras GC→AT mutations and tumors induced by methylating agents.[116] 7-mGua is rapidly removed by base excision repair (BER) as well as by spontaneous depurination.[116] The latter gives rise to apurinic sites that are prone to undergo rapid and error-free repair.[116] In contrast to O6-mGua, 7-mGua seems to have low mutagenic potency, because there was no correlation between persistence of 7-mGua adduct levels from NNK and incidence of liver tumors in rodents.[116] O6-pobdG has been shown to be efficiently repaired by AGT both in vitro and in vivo.[116] If not repaired, O6-pobdG adducts induce large numbers of G→A and G→T mutations.[116]
Experimental data has suggested that a multistep process of genetic alterations is responsible for NNK- and NNN-induced carcinogenesis.[116] DNA adducts that are mis-repaired or not repaired constitute a necessary, although not sufficient, prerequisite for induction of cancer.[116] Initiation and progression of tumorigenesis, however, is complex and involves inactivation of tumor suppressor genes, activation of oncogenes, inflammatory processes as well as alterations in the tissue microenvironment.[116] Susceptibility depends in part on the balance between carcinogen metabolic activation and detoxification in the nicotine users.[116] The genetic polymorphisms in carcinogen-activating genes as well as in DNA repair genes are important determinants of DNA-adduct levels.[116] DNA repair system sets up the second defense line required for eliminating or repairing the lesions of DNA adducts in the genome from the insults of NNK or NNN.[116]

Oxidative stress occurs when the productions of oxidant species (mostly reactive oxygen species and reactive nitrogen species exceed the cellular neutralizing capabilities.[116] The mitochondrial respiratory chain generates the majority of reactive oxygen species in aerobic cells by incomplete reduction of molecular O2 to H2O during oxidative phosphorylation, as well as during microsomal and peroxisomal oxidations.[116] In addition, the production of reactive oxygen species and reactive nitrogen species are also associated with a number of processes such as inflammation, infections, and immune reaction.[116]
The mechanisms of NNK- and NNN-induced oxidative stress are not well understood.[116] However, the ability of NNK to induce oxidative stress was evident when increasing levels of 8-Oxo-2'-deoxyguanosine adducts in lung tissues were detected after either oral administration or intraperitoneal injection of NNK into A/J mice and rats.[116] 8-Oxo-2'-deoxyguanosine is a major pre-mutagenic lesion generated from reactive oxygen species that is considered a marker of DNA oxidative damage.[116] 8-Oxo-2'-deoxyguanosine is removed by Mmh/Ogg1 gene product, 8-hydroxyguanine DNA glycosylase 1 (OGG1) through the BER pathway.[116] Although NNK-mediated reactive oxygen species induce DNA lesions, another important aspect is reactive oxygen species-mediated alteration of the microenvironment required for tumor progression.[116] Reactive oxygen species act as signaling intermediates for many normal as well as pathological cellular processes.[116] Constant activation of transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) appears to be one functional role of elevated reactive oxygen species levels during tumor progression.[116]
Genotoxicity and the tumor-promoting environment are two essential conditions for tobacco specific nitrosamines-induced cancer.[116] It has been shown that the simultaneous expression of oncogenic K-ras, p53 knockdown, and mutant EGFRs were insufficient to confer a full malignant phenotype in bronchial epithelial cells.[116] NNK induces nearly identical numbers of mutation and comparable levels of mutagenic DNA adducts in both susceptible and resistant lungs suggesting a pro-tumor environment is essential for tumor progression.[116] The upregulation of nicotinic acetylcholine receptors and concomitant desensitization of alpha-4 beta-2 nicotinic receptor in nicotine users shifts the balance in favor of alpha-7 nicotinic receptor signaling with strong direct and indirect stimulatory effects on cancer cells, whereas the release of GABA, which counteracts many of these effects, is reduced.[116] This universal switch from balanced neurotransmission to cancer-stimulating neurotransmission is unstoppable once it occurs.[116] Blocking one signaling pathway or even removing the primary cancer will not stop the runaway alpha-7 nicotinic receptor activity.[116]
Volatile organic compounds
A 2018 study found that e-cigarette–only users had up to three times urinary levels of five volatile organic compounds, and there were volatile organic compounds that were considered carcinogenic, and they were present whether the product contained nicotine or flavorings.[114]
California Proposition 65
Acetaldehyde, benzene, cadmium, formaldehyde, isoprene, lead, nickel, nicotine, NNN, and toluene[39] are on the California's Proposition 65 list of chemicals known to the state to cause cancer, birth defects, or other reproductive harm.[40] Delta-9-tetrahydrocannabinol (as well as cannabis smoke[126]) is on the California's Proposition 65 list of chemicals known to the state to cause developmental harm and cancer.[127]
California Proposition 65 requires businesses to determine if they must provide a warning about significant exposure to listed chemicals.[128] Businesses with ten or more employees that expose individuals to listed chemicals through their products or operations generally must provide warnings.[129]
Specific body parts
Bladder
E-liquids have been found to include aromatic amines, aldehydes, and polycyclic aromatic hydrocarbons, all of which have been found to cause bladder cancer in humans.[19] Benz(a)anthracene and benzo(a)pyrene, aromatic amines, and aldehydes, among other bladder carcinogens, have been found in e-cigarette liquids, aerosol, or urine in previous research.[19] The use of e-cigarettes increases the odds of being diagnosed with bladder cancer.[1] The long-term impact of persistent exposure to carcinogens such as polycyclic aromatic hydrocarbons, volatile organic compounds, and tobacco-specific nitrosamines in the urinary tract epithelium among those who use e-cigarettes over a long period of time remains unclear.[130]
A 2018 case report found that the levels of the bladder cancer-causing chemicals 2-naphthylamine and o-toluidine in the urine of e-cigarette users are higher when compared to non-smoking, non-e-cigarette-using controls.[19] Before supplying samples for this investigation, the majority of these subjects had not smoked a typical cigarette in over a year.[19] This research raises the possibility that using an e-cigarette with varying liquid and aerosol control formulas may not be completely risk-free from the perspective of bladder cancer.[19] 2-naphthylamine and o-toluidine are suspected to act as bladder carcinogens in humans.[64]
The 2018 case report describes a 16-year-old girl who developed hypersensitivity pneumonitis after using e-cigarettes for several months.[19] The person presented with symptoms such as cough, shortness of breath, and fever, and was diagnosed with hypersensitivity pneumonitis based on clinical and radiographic findings.[19] The authors suggest that e-cigarette use may have contributed to the development of hypersensitivity pneumonitis in this person, highlighting the potential risks associated with e-cigarettes on lung health.[19] To fully comprehend the effects of e-cigarettes, use on lung health, more research is required.[19]
A 2018 study showed that the nitrosamines and downstream metabolites of nicotine present in e-cigarettes put e-cigarette users at greater risk than non-users for developing lung or bladder malignancies or heart disease.[19] The 2021 case report shows in contrast to non-e-cigarette users, those who use e-cigarettes have higher levels of carcinogens that can be metabolized into several compounds that can cause bladder cancer, which can be identified by urine sampling.[19]
Brain
The e‑cigarette aerosol is absorbed through the lungs, and at this point it rapidly travels through the heart and subsequently delivers nicotine to the brain within a matter of a few seconds.[131] Nicotine in the brain of e-cigarette users is typically between 0.05 and 0.5 μM.[132] Nicotine helps facilitate brain metastasis.[133]
Breast
Breast cancer is the cancer that affects women most frequently in the US, accounting for almost one-third of all cancer diagnoses in this population and more than 18 to 20% of all cancer-related deaths in women.[19] There is evidence that e-cigarettes promote lung metastasis of human breast cancer cells.[19] This is an important contribution to understanding the potential risks that e-cigarettes pose to human health.[19]
Previous research has shown that e-cigarette use increases lung carcinogenesis by causing the production of DNA adducts in the lungs.[19] Additionally, a 2018 case report demonstrates that inhaling e-cigarettes may cause the release of oncogenic cytokines or microRNA from both pulmonary cells and breast cancer cells, promoting lung colonization of breast cancer cells like the colonization of conventional chondrosarcoma cells, which promotes the metastasis of breast cancer-causing breast cells.[19]
A 2020 study in the context of e-cigarette-enhanced breast cancer development and metastasis, evaluates the crucial involvement of myeloid cells and related signaling pathways.[19] The microenvironment of every organ in the body is typically tumor-suppressive under physiological circumstances.[19] However, a tumor-promoting microenvironment can develop as a result of persistent inflammation brought on by a variety of causes.[19]
In a 2020 study, e-cigarette inhalation, similar to conventional cigarettes, may induce the release of oncogenic cytokines or microRNAs from both lung and breast cancer cells, thereby promoting lung colonization by breast cancer cells.[19] A third possibility is that exposure to e-cigarettes may improve the survival of breast cancer cells during the invasion and nesting process.[19] Evidence from the literature suggests that cancer cells are prone to apoptosis during metastasis.[19]
Cervix
Nicotine triggers a multitude of effects on cervical cancer cells.[24]
Colon
The use of alcohol with nicotine increases the likelihood of getting colorectal cancer.[134] NNK stimulated the growth of colon cancer cells by increasing the mRNA expression of the alpha-7 nicotinic receptor and by amplifying the binding interaction of nuclear factor-kappa B.[107]
Connective tissue
Nicotine can stimulate muscle sarcomas in A/J mice.[135] In animal studies, nicotine alone causes sarcomas and leiomyomas.[42]
Gastrointestinal tract
Through a range of mechanisms, nicotine promotes the growth of gastric cancer cells.[24] NNK, through its carcinogenic effects, can promote the development of gastrointestinal cancers.[107] Its mechanism of action in inducing gastrointestinal cancer is not well understand.[136]
Head and neck
Studies using different brands of e-cigarette aerosol with or without nicotine, as well as heavy metals like cadmium, lead, nickel, and nitrosamines showed decreased cell viability and apoptosis compared to unexposed controls and significant evidence of necrosis in head and neck squamous cell carcinoma and normal epithelial cell lines.[19] Additionally, exposed cell lines expressed increased histone H2A family member X (H2AX), a recognizable indicator of double-stranded DNA breakage.[19]
A 2016 case report reported significant DNA double-strand breaks being induced in cells exposed to e-cigarette (0.5 to 2% volume of e-cigarette aerosol ranging from 24 hours to 4 weeks) aerosols as well as an increase in the migration of head and neck cancer cells after e-cigarette treatment with upregulation of epithelial-to-mesenchymal transition-promoting genes.[19]
The 2017 case report describes a 59-year-old man who developed a basaloid squamous cell carcinoma after using 30 e-cigarettes every day for the previous 13 years.[19] The authors suggest that e-cigarette use may have contributed to the development of basaloid squamous cell carcinoma, highlighting the potential risks associated with long-term e-cigarette use.[19] The same 2017 case report describes a 66-year-old man who developed a basaloid squamous cell carcinoma after using e-cigarettes 20 times every day for the previous 13 years.[19] The authors suggest that e-cigarette use may have contributed to the development of basaloid squamous cell carcinoma, highlighting the potential risks associated with long-term e-cigarette use.[19]
The 2021 case report describes a 19-year-old man who developed a nonhealing left lateral tongue ulcer later found as a stage IV tumor after using e-cigarettes (0.5 packs) each day for four years.[19] The person used vaping daily nicotine-delivery systems (Juul) and had no history of tobacco smoking.[19] The authors suggest that e-cigarette use may have contributed to the development of cancer, which highlights the potential risks associated with long-term e-cigarette use.[19]
There is plenty of evidence from in vitro, in vivo, and human cohort studies that shows acrolein's cancer potential.[66] Moreover, the research shows that acrolein, by affecting specific signaling pathways, is not only directly involved in mutagenesis but also contributes to increasing the resistance of cancer cells to traditional cisplatin chemotherapy.[66] Clear evidence for the carcinogenic and cytotoxic properties of acrolein is provided by the studies of Matsumoto et al., where a 2-year inhalation of acrolein in both mice and rats induced, among others, squamous cell carcinomas in the nasal cavity.[66]
Lungs
Vaping exposes the lungs to a variety of chemicals, including those added to e-liquids, and other chemicals produced during the heating or aerosolizing process.[138] Although the association between vaping and the development of lung cancer is not well established, the carcinogenicity of breathing in of substances such as nitrosamine compounds, humectants (propylene glycol and glycerin), flavoring compounds, cannabis, and vitamin E acetate has been attributed to several possible mechanisms.[75]
Inflammation is considered a primary cause of various types of cancer, including lung cancer.[32] Exposure to e-cigarette aerosol may trigger inflammation in the airways, which is a risk factor for the development of lung cancer.[32] Particles containing formaldehyde, acetaldehyde, and reactive oxygen species can form deposits in the bronchioles or alveoli and cause inflammatory damage of the respiratory epithelium.[32]
A 2017 study that utilized a rat model for investigating lung cancer had demonstrated various effects of e-cigarette aerosol on initiating cancer.[109] Specifically, higher production of CYP that facilitates the metabolic activation of polycyclic aromatic hydrocarbons from exposure to e-cigarette aerosol is associated with the development of lung cancer in rats.[109] These enzymes were connected to the excessive production of reactive oxygen species and subsequent oxidation of DNA, which culminated in the formation of 8-Oxo-2'-deoxyguanosine.[109] Additionally, the same study found that e-cigarette aerosol triggered damage to the DNA in peripheral blood.[109] This was shown by fragmented DNA and strand breaks in leukocytes, which was accompanied by the emergence of micronuclei in reticulocytes.[109]
Nicotine exposure can instigate the development of cancer stem cell-like properties in non-small cell lung cancer, a process known as stemness.[109] This process is linked to the use of e-cigarettes and involves the increased expression of the SRY (sex determining region Y)-box 2 (Sox2) gene, which is an important stemness marker that is critical for the self-renewal of stem cells.[109] Moreover, this induction of stemness is further driven by activating the Yes associated protein 1/ E2F transcription factor 1/Octamer-binding transcription factor 4 (Yap1/E2F1/Oct4) signaling pathway.[109]
The 2017 case report describes a 51-year-old female who developed breast cancer after using e-cigarettes.[19] Since she believed e-cigarettes were safer than regular cigarettes, she switched to them around three months before her operation and continued to use them at a rate equivalent to her previous one and a half packs per day.[19] The authors suggest that e-cigarette use may have contributed to the development of breast cancer, highlighting the potential risks associated with long-term e-cigarette use.[19]
A 2016 case report demonstrates that e-cigarette usage (38 mg/mL, 10 mL per week) caused severe liver and lung inflammation in a 45-year-old patient, simulating metastatic disease.[19] Results showed that e-cigarette use promoted epithelial-to-mesenchymal transitions and interfered with DNA repair mechanisms, which supported the link between e-cigarette use and the progression of cancer.[19]
In the 2017 case study it was discovered that e-cigarettes contain nicotine and its metabolites as well as a small amount of nickel in the users' saliva, urine, and exhaled breath.[19] Although they do not smoke tobacco, they nevertheless run the danger of developing lung cancer from the nicotine and nickel in e-cigarettes.[19]
Liver
Nicotine enhances liver cancer cell growth.[24]
Mouth and throat
There is a lack of research on the effects of e-cigarettes related to oral cancer.[139] No compelling evidence from vaping indicates it directly causes oral cancer,[140] though several case studies have reported oral cancer development in people with a history of vaping.[20] E-cigarette use may trigger a variety of potentially carcinogenic events at the molecular level in oral cells.[139]
Pancreas
Nicotine increases the metastatic potential of pancreatic cancer cells.[24]
Stomach
Long-term nicotine use can initiate the onset of stomach tumors.[107]
Bystanders
Environmental e-cigarette aerosol exposure


Environmental concerns and issues regarding non-user exposure exist.[64] Substantial amounts of aerosol and nicotine are released into indoor air following their use.[21] These products cannot be considered safe for second-hand exposure, as the e-cigarettes emits the finest particles of liquid nicotine and carcinogens into the air and consequently can lead to inhaling them.[64] Although e-cigarette companies state that e-cigarette aerosols are merely water vapor, propylene glycol, and glycerin, and is safe to use anywhere, research has proven that indoor nicotine, fine and ultrafine particulate matter, polycyclic aromatic hydrocarbons, metals such as aluminum, and certain volatile inorganic compounds increase after the aerosol is exhaled.[55]
Bystanders can inadvertently be exposed to the e-cigarette second-hand and third-hand aerosols.[27] Although it may not be as dangerous as second-hand smoke from classical cigarettes, people passively exposed to e-cigarette aerosol absorb nicotine at levels comparable to passive smokers.[39] They are also exposed to volatile organic compounds and fine and ultrafine particles.[39] These ultrafine particles can travel deep into the lungs and lead to tissue inflammation.[39] The low parental perception of the risks connected to e-cigarette exposure for children increases their susceptibility to harmful effects from passive vaping.[114]
Second-hand exhaled exposure to nicotine and cancer-causing chemicals in indoor places may result in serious unwelcomed effects.[28] Surfaces can become polluted with nicotine from the use of e-cigarettes indoors.[141] Nicotine from aerosols or e-cigarette liquids remains on surfaces for weeks or months and reacts with the environment to form nitrates and tobacco-specific nitrosamine compounds, which can to lead inhalation, ingestion, or dermal contact with carcinogens.[19]
E-cigarettes are commonly used in many places, such as homes, cars, restaurants, bars, and workplaces, where vulnerable populations, such as children, adolescents, and pregnant women, might be exposed.[30] Second-hand exposure in indoor environments is of particular concern because people typically spend more than 80% of their time indoors, where emitted pollutants are not diluted as quickly or as extensively as outdoors.[30] Children, particularly young children, may be exposed to the developmental toxicant nicotine from indoor surfaces long after someone had been vaping.[29] The evidence indicates that the utmost caution should be exercised when it concerns children being exposed to nicotine and other developmental toxicants.[29]
Exhaled e-cigarette aerosols, containing substances such as nicotine and carcinogenic alkaloids, that originated from a vape shop was detected in a neighboring business in 2018.[142] The second-hand aerosols constituents from a vape shop traveled to form deposits on indoor surfaces in an adjacent business.[143]
Environmental tobacco smoke exposure
The 2004 Tobacco Smoke and Involuntary Smoking book states that the International Agency for Research on Cancer has determined "that involuntary smoking (exposure to secondhand or 'environmental' tobacco smoke) is carcinogenic to humans."[144] The Centers for Disease Control and Prevention states that commercial tobacco smoke contains hundreds of harmful chemicals and about 70 of them in commercial tobacco smoke can cause cancer.[145] Chemicals and toxicants in commercial tobacco smoke include benzene, toluene, butane, cadmium, ammonia, and hydrogen cyanide.[145] Nicotine is potentially harmful to non-users.[146]
There is no safe level of exposure to second-hand smoke.[145] Even brief exposure can cause serious health problems.[145] Second-hand smoke can cause coronary heart disease, stroke, and lung cancer in adults who do not smoke.[145] Because their bodies are still growing, infants and young children are especially impacted by health problems caused by second-hand smoke.[145] Children of smoking parents are up to 13 times more likely to be exposed to second-hand smoke.[147] Eliminating smoking is the only way to fully protect people from second-hand smoke exposure.[145] The harm caused by second-hand smoke is preventable.[145]
Children are uniquely susceptible to toxic aerosols, including cigarette smoke especially due to second-hand smoke or environmental tobacco smoke.[85] This vulnerability is because of three major reasons: (a) children have disproportionately heavier exposures in relation to body weight than adults for the same amount of toxic aerosols; (b) children are extremely sensitive to these exposures, lacking the ability to metabolize, detoxify and excrete those toxic compounds; and (c) especially small children often reside very close to their parents who may be smokers.[85] The statistics show that in the US, more than 20% children live with smokers, where especially children living in public housing units endure much higher second-hand smoke-exposure than the national average.[85]
Environmental heated tobacco product emissions exposure
Heated tobacco product emissions have been reported to increase the levels of acetaldehyde, benzene, formaldehyde, nicotine, toluene, and particulate matter in a range of indoor environments.[148] Exposure to considerably greater contaminate levels of benzene, formaldehyde, and toluene could happen in public places.[148]
While animal studies, and human clinical studies by Philip Morris International researchers claim that IQOS aerosol is significantly less harmful to human health than classical cigarette smoke, findings from independent reviews of Philip Morris International's own data shows that IQOS aerosol is as harmful as classical cigarette smoke to human health.[37] Significant levels of n-alkanes, organic acids, and carcinogenic aldehydes including formaldehyde, acetaldehyde, and acrolein have been observed in IQOS side stream aerosol.[37]
People diagnosed with cancer
Nicotine can stimulate the sympathoadrenal system.[52] It can increase the secretion of norepinephrine and epinephrine by stimulating various mechanisms of the sympathoadrenal system.[52] The underlying mechanisms include the following: stimulating the nerve endings in sympathetic nerves directly; activating the nicotinic acetylcholine receptors on the cell bodies of sympathetic postganglionic neurons; and engaging the central nervous system structures that control the sympathetic outflow.[52]
Certain types of cancers appear to exhibit a heightened responsiveness to the stimulative impact of the sympathoadrenal system.[52] The stimulation of the sympathoadrenal system resulting from the use of nicotine-enriched e-cigarettes may play a crucial role in the advancement of cancer in people who have cancer.[52]
People with a genetic predisposition to cancer
The use of e-cigarettes could pose risks for people who are predisposed to getting cancer.[52]
Prevalence among people diagnosed with cancer
The increased usage of e-cigarettes among people diagnosed with cancer could be associated with the belief that they are a less dangerous option when compared against classical cigarettes..[52]
Perception among people diagnosed with cancer
Emerging preclinical research suggests that nicotine vaping can activate the sympathetic nervous system, which may promote cancer development and growth through various mechanisms.[52] This concern could be particularly relevant for people diagnosed with cancer who are undergoing medical treatment, as they may falsely believe that e-cigarettes are a safe option when judged in contrast to classical cigarettes.[52]
Nicotine cessation
The American Cancer Society states that the best option is to "stop using all tobacco products, including e-cigarettes, as soon as possible both to reduce health risks and to avoid staying addicted to nicotine."[149] The Centers for Disease Control and Prevention states that adults who switch to e-cigarettes should also establish a goal for quitting them, to fully eliminate health risks from any tobacco product use.[150] The American Lung Association states that "Despite what Juul and e-cigarette companies want you to believe, switching to vaping (e-cigarettes) is not quitting smoking. E-cigarettes are still tobacco products, and FDA has not approved any e-cigarette as a quit smoking device."[151] E-cigarette companies are making unsupported health claims that are "confusing people who want to quit smoking."[151]
Tobacco control
Over the years, tobacco control programs and interventions have demonstrated significant success in decreasing initiation in non-smokers and cessation in smokers.[43] However e-cigarettes may reverse this success in smoking cessation.[43] E-cigarette users may perceive the device as a useful alternative to traditional tobacco smoking.[43]
With the advent of e-cigarettes and common positive perceptions regarding their use, the world is at risk of reversing the years of efforts regarding tobacco control and instead advance towards a new addiction with, as of 2022, unknown long-term health hazards.[43]
Public health
Over the past ten years leading up to 2020, the dramatic increase in their usage has led the medical community to evaluate their potential harms to health.[56] Among these risks, the potential for cancer development has been a significant concern.[56]
With a wide range of formulas, e-cigarettes have historically been uncontrolled[19] and usually lack manufacturing standards.[28] Poor regulation impacts safety.[28] There is growing evidence that e-cigarettes cause harm to children.[57] Because of this, a 2018 review cautions against its public advocacy and its usage.[57]
The use of e-cigarettes has been recognized as a global public health problem.[152] The widespread use of e-cigarette among minors endangers the public health accomplishments that have been effective in deglamorizing and diminishing the consumption of tobacco products.[153] The health care costs caused by the negative effects of nicotine are staggering.[154]
While carcinogens, such as nitrosamines, induce cancer by causing gene mutations and/or DNA and protein adducts, nicotine promotes cancer progression by activating signaling pathways that facilitate cancer cell growth, angiogenesis, migration, and invasion.[116] The nicotine and carcinogen alliance is detrimental to human health, costs billions in direct medical care, causes loss of productivity, and is responsible for millions of preventable and premature deaths each year.[116]
It is worth fearing that wide-scale promotion and use of e-cigs, fuelled by an increase in the advertising of these products, may carry substantial public health risks. Indeed, nonsmokers may start using e-cigs because they have heard it is less harmful than traditional tobacco rather than remaining naïve of smoking which is by far the best attitude. Besides, e-cigs may serve as a gateway product, that young people who first experiment with these products will move on to traditional tobacco use. Further, normalization of e-cig use may lead former cigarette smokers to begin using this new device, thereby reinstating their nicotine dependence and fostering a return to tobacco use.[155]
— Jobert Richie N Nansseu and Jean Joel R Bigna, Pulmonary Medicine[155]
Tobacco companies and undermining and distorting science
Faced with irrefutable, peer-reviewed proof of smoking’s detrimental effects, the tobacco industry started a campaign in the 1950s that engaged in elaborate public relations strategies to discredit and warp the mushrooming scientific data.[156] Beginning in December 1953, tobacco companies would adapt a cohesive stance on smoking and health, which would usher in an era spanning more than five decades of intentional and overt collaboration.[156]
By the early 1960s, even with overwhelming research showing the dangers of smoking, a substantial "controversy" had commenced, which was orchestrated by the tobacco industry in regard to the credibility and interpretation of these research findings.[156] In 1963, Joseph Lelyveld, quoting an unspecified American Cancer Society representative, wrote in an article published in The New York Times: "Surprisingly, the furor over smoking and health failed to send the industry into a slump. Instead, it sent it into an upheaval that has resulted in unforeseen growth and profits. When the tobacco companies say they're eager to find out the truth, they want you to think the truth isn't known…. They want to be able to call it a controversy."[156] Biased research paid for by the tobacco industry continues to be widespread in the field of e-cigarettes, as of 2019.[157]
Tobacco companies and regulatory interference
Big tobacco companies have worked with organizations, such as the e-cigarette association, consumer advocates for smoke-free alternatives association, and Vapers International Inc., to delay or eliminate legislation aimed at limiting e-cigarette use and sales.[43] A 2019 review states, "The tobacco industry continues its relentless pursuit of profit using well-funded and well-rehearsed strategies. It is applying the lessons learned from 100 years of cigarette marketing and counter-control propaganda to cast doubt, confuse, divide and misdirect e-cigarette regulation, seeking to recruit new generations of smokers and nicotine addicts."[157]
Regulatory impact on e-cigarette usage
The rapid increase in e-cigarette use among young people has been a global public health challenge, given the potential harm of e-cigarettes and nicotine dependence.[158] Many countries have recently, as of 2023, introduced legislations to regulate e-cigarettes, but the impacts of these policies are poorly understood.[158]
Flavor restrictions were found to significantly decrease youth e-cigarettes use, and taxation reduced adult use; mixed results were found for the impacts of age restrictions.[158] Flavor restrictions and taxes are backed up by the strongest evidence for effectively regulating the usage of e-cigarettes, while other regulatory measures require strict enforcement and meaningful penalties in order to be able to maintain their intended outcomes.[158]
Potential mechanisms of nicotine on lung cancer progression
Nicotine may induce α7nAChR expression in human SCLC cells via the Sp1/GATA regulation signaling pathway.[124] α7nAChR expression levels are elevated in SCC compared with adenocarcinoma of the lung.[124] High α7nAChR expression levels in lung cancer cells may be involved in the nicotine-induced tumorigenesis.[124] α7nAChR levels in patients with SCC who are active smokers are correlated with their smoking history.[124] The function of α7nAChR-mediated lung cancer progression including in proliferation, angiogenesis, and metastasis, has been revealed.[124]
Cell proliferation
α7nAChR mediates the proliferative effects of nicotine in lung cancer cells.[124] Nicotine/α7nAChR signaling enhances NSCLC cell proliferation by scaffolding protein β-arrestin-mediated activation of the Src and Rb-RAF protooncogene serine/threonine-protein kinase (RAF-1) pathways.[124] Moreover, continuous exposure to nicotine in SCC of the lungs results in α7nAChRs upregulation, which may enhance tumor growth.[124] Nicotine stimulates tumor growth and ERK activation in a murine orthotopic model of lung cancer.[124] A blockade of α7nAChRs suppresses nicotine-induced lung cancer cell growth and vimentin expression through the MEK/ERK signaling pathway.[124] Consistent with these results, nicotine increased expression of nAChR and stimulated proliferation of SCC cell line.[124] α7nAChR may mediate the proliferative activity of nicotine in poorly differentiated NSCLC.[124] Nicotine-induced α7nAChR and α4nAChR expression in NSCLC cells, along with p-CREB and p-ERK1/2 activation accompanied by increased noradrenaline, leading to cell proliferation.[124] Nicotine/α7-nAChR promoted proliferation in human SCLC cells via the Sp1/GATA regulation signaling pathway.[124] NNK/α7nAChR may increase cell growth in SCLC cells via an influx of Ca2+.[124] Nicotine stimulates NSCLC cell proliferation and PPARβ/δ expression through activation of PI3K/mTOR signals that suppress AP-2α binding activity to PPARβ/δ promoter.[124] α7nAChRs or α9nAChRs mediated nicotine-induced cell proliferation and activation of the AKT and ERK signaling pathways.[124] The activated cell-membrane α7nAChRs formed complexes with EGFR, whereas activated mitochondrial α7nAChRs was physically associated with the intramitochondrial protein kinases PI3K and Src that increased expression of cyclin D1 and activation of ERK1/2 lead to lung cancer proliferation.[124] α-Cobratoxin, a high-affinity α7-nAChR antagonist reduced tumor growth in nude mice orthotopically engrafted with NSCLC cells.[124] An α7nAChR inhibitor (APS8) may suppress NSCLCs proliferative effects of nicotine.[124] These studies revealed that nicotine/α7nAChR signals mediate proliferation in lung cancer.[124]
Metastasis
Cigarette smoke status and history are associated with lung cancer metastasis.[124] α7nAChRs may mediate cancer cell growth depending on NSCLC differentiation status.[124] α7nAChR and heteromeric nAChRs can promote tumor invasion in NSCLC.[124] Nicotine/α7-nAChR can induce NSCLC cell migration and invasion via the MEK/ERK signaling pathway.[124] NNK promotes lung cancer cell migration and contactin-1 expression via the α7nAChR-mediated ERK signaling pathway.[124] Moreover, NNK enhances lung cancer cell migration and invasion via activation of the c-Src-PKCiota-FAK signaling axis.[124] β-Cryptoxanthin may repress lung cancer cell motility through the downregulation of α7nAChR/PI3K signaling.[124] Nicotine/α7nAChR signaling enhance migration and the expression of SOX2 in NSCLC cell lines through the YAP-E2F1 signaling axis.[124] These studies suggest that α7nAChR can enhance lung cancer cell metastasis through the activation of different signaling pathways.[124]
Angiogenesis
α7nAChR enhances angiogenesis via the PI3K/AKT pathway and NF-κB activation, which is partially dependent on vascular endothelial growth factor (VEGF).[124] Nicotine/α7nAChR signaling mediates proangiogenic effects through angiogenesis and EMT.[124] MG624, an α7nAChR antagonist, reduces nicotine-induced early growth response gene 1 (Egr-1) binding activity to the fibroblast growth factor 2 (FGF2) promoter that inhibits angiogenic effects in SCLC 66.[124] Thus, α7nAChR may facilitate lung cancer progression including angiogenesis; however, its detailed effect requires investigation.[124]
Anti-Inflammation
α7nAChR attenuates ventilator-induced lung injury and plays an anti-inflammatory role in several inflammatory diseases.[124] Activation of inflammation‐related receptors, such as toll-like receptors, enhances NF-κB signaling pathways in both acute and chronic inflammation that can considerably increase cancer risk.[124] Choline/α7nAChR signaling modulates TNF release via the inhibition of NF‐κB activation.[124] Moreover, nicotine/α7nAChR mediates anti-inflammatory action on macrophages via recruitment and activation of JAK2, initiating the STAT3 and SOCS3 signaling cascade.[124] Nicotine/α7nAChR suppresses TNF-α expression in human airway epithelial cells by inhibiting MyD88 and NF-κB activity.[124] An anti-inflammation study revealed that α7nAChR signaling inhibits NLRP3 inflammasome activation by preventing mitochondrial DNA release.[124] Accumulating evidence suggests that the vagus nerve may modulate lung infection and inflammation through the α7nAChR signaling pathway.[124] Thus, α7nAChR may be a potential target for attenuating inflammatory cytokine production in lung diseases.[124]
Exposure to nicotine adversely affects dendritic cells, a cell type that has an important role in anticancer immunosurveillance.[124] Nicotine may suppress anticancer immunity by increasing or reducing number of regulatory T cells and T helper 17 cells, respectively.[124] Moreover, nicotine inhibits the cytotoxic activity of natural killer (NK) cells, and the effect of nicotine on NK cells can be abolished by β2nAChR deficiency.[124] However, to understand immune regulation and progression in lung cancer, the roles of nicotine-mediated pathways in distinct immune cells warrant investigation.[124]
Potential mechanisms of nicotine and sirtuins on drug resistance
Nicotine may reduce the cytotoxic effects of chemotherapy and radiotherapy that cause poor therapeutic response.[124] Nicotine-mediated tumor-promoting effects are apparently mediated by nAChRs expressed on cell membranes and by mitochondria.[124] Additionally, mitochondria are critical mediators of cancer progression, as this process requires flexibility to adapt to cellular and environmental alterations in addition to cancer therapies.[124] Nicotine-impaired metabolism and mitochondrial defects were critical in metabolic responses to cancer progression.[124] Activation of cell-membrane and mitochondrial nAChRs produces a combination of growth-promoting and antiapoptotic signals that implemented the tumor-promoting action of nicotine in lung cells.[124] Furthermore, nAChRs were identified to control either CaKMII or Src-dependent signaling pathways in mitochondria that protect cells from apoptosis.[124]
Nicotine also permeated cells and activated mitochondrion-nAChRs coupling to inhibit mitochondrial permeability transition pore (mPTP) opening, preventing apoptosis.[124] Nicotine-induced survival may occur by a mechanism of multisite phosphorylation of BAD, which may lead to human lung cancer and/or chemoresistance development.[124] Activation of nicotine-α7nAChR signaling can trigger membrane depolarization, which activates voltage-gated calcium channels and subsequently activates the MAPK pathway, possibly increasing B-cell lymphoma-2 (Bcl-2) expression and apoptosis downregulation.[124] Nicotine prevents cisplatin-mediated apoptosis by regulating α5nAChR/AKT signaling and several mitochondria proteins including Bcl-2, Bax, survivin, and caspase 3 in gastric cancer cells.[124] Long-term nicotine exposure-induced chemoresistance is mediated by STAT3 activation and ERK1/2 downregulation via nAChR and β-AR in bladder cancer cells.[124] Emerging evidence suggests that feedback activation of STAT3 signaling is a common cause of drug resistance to receptor tyrosine kinase-targeted therapies and conventional chemotherapy.[124] Long-term exposure to NNK combined with arecoline activated EGFR/AKT signaling is involved in antiapoptosis, cancer stem cell properties, and cisplatin resistance in head and neck SCC (HNSCC) cells.[124] Nicotine/α9nAChR-PPM1F signaling can attenuate p-p53 (Ser-20)- and p-BAX (Ser-184)-induced proapoptotic pathways.[124] Therefore, nAChRs may be a promising molecular target to arrest lung cancer progression and reopen mitochondrial apoptotic pathways.[124] Nicotine can induce erlotinib resistance via the crosstalk between α1nAChR and EGFR/AKT/ERK signaling pathways in NSCLC.[124]
Sirtuins can exert their capacity to respond to environmental changes and their expression is often altered in cancer.[124] SIRT1, SIRT3, SIRT4, and SIRT7 are strongly expressed in lung adenocarcinoma, whereas SIRT5 is highly expressed in SCC.[124] Analysis of the TCGA NSCLC dataset revealed that high expression levels of SIRT2/6 were associated with longer overall survival (OS).[124] However, high SIRT6 expression was associated with poor OS in 98 patients with NSCLC.[124] Nicotine enhanced oxidative stress and activates NF-κB.[124] Several sirtuins are critical in inhibiting excessive, damaging levels of reactive oxygen species that drive cancer drug resistance.[124] Nuclear SIRT1 promotes reactive oxygen species stress resistance via the deacetylation of several transcriptional regulators, including p53, forkhead homeobox type O (FOXO) proteins, PGC-1α, heat shock factor protein 1 (HSF1), and nuclear erythroid factor 2-related factor 2 (NRF2), and this contributes to antioxidant production.[124] Studies have suggested that mitochondrial-sirtuins (SIRT3, SIRT4, and SIRT5) are members of a family of NAD+-dependent deacetylases and are implicated in the oxidative stress response through the regulation of mitochondrial metabolism and antioxidant mechanisms.[124] Mitochondrial SIRT3 may coordinate ROS; SIRT5 also limits reactive oxygen species by activating SOD1 and NRF2 to maintain cellular redox homeostasis.[124] Thus, sirtuins may promote cancer cell survival by limiting reactive oxygen species that would lead to cancer drug resistance.[124] Several recent studies have supported the presence of sirtuins-mediated drug resistance with human cancers.[124] Some sirtuins regulate lung cancer progression and signaling molecules are associated with drug resistance.[124]
SIRT1
Nicotine can upregulate SIRT1 expression in a time- and concentration-dependent manner.[124] BaP, a carcinogen in cigarette smoke, can induce SIRT1 in human bronchial epithelial cells.[124] SIRT1 is involved in BaP-induced transformation associated with TNF-α-β-catenin axis activation and is a potential therapeutic target for lung cancer.[124] α7nAChR-SIRT1 axis activation alleviates angiotensin II-induced VSMC senescence.[124] Recent studies, as of 2020, have focused on the biological functions of SIRT1 in metabolic diseases, cancer, aging and cellular senescence, inflammatory signaling in response to environmental stress, and cell survival.[124] SIRT1 expression was a strong predictor for poor OS and progression-free survival in patients with NSCLC who underwent platinum-based chemotherapy.[124] Silencing of SIRT1 could significantly enhance the chemosensitivity of lung cancer cells to cisplatin treatment.[124] SIRT1 was negatively associated with proapoptotic factors BAD, BAX, and BID in TCGA NSCLC patients.[124] SIRT1 suppression sensitizes lung cancer cells to WEE1 inhibitor-induced DNA damage and apoptosis.[124] SIRT1 deacetylates and inactivates p53, allowing cells to bypass apoptosis.[124] The transcription factor FOXO 3 alpha (FOXO3a) may induce the expression of several antioxidant genes, including Mn superoxide dismutase (MnSOD), catalase, peroxiredoxins 3 and 5 (Prx3 and Prx5, respectively), thioredoxin 2 (Trx2), thioredoxin reductase 2 (TR2), and uncoupling protein 2 (UCP-2).[124] SIRT1-mediated deacetylation of FOXO3a increases cell survival in response to oxidative stress.[124] Moreover, SIRT1 may play a role in the acquisition of aggressiveness and chemoresistance in ovarian cancer and have potential as a therapeutic target for ovarian cancer.[124]
SIRT2-SIRT7
SIRT3, located in mitochondria, is correlated with NSCLC malignancy.[124] α7nAChRs activation inhibits platelet-derived growth factor‐induced cells migration by activating the mitochondrial deacetylase SIRT3, implying a critical role for α7nAChRs in mitochondrial biology and PDGF‐related diseases.[124] The activity of SIRT3 can protect cancer cells from chemotherapy-induced oxidative stress.[124] Additionally, SIRT3 promotes the activation of AKT signaling pathways in NSCLC.[124] SIRT3 promoted p53 degradation in PTEN-deficient NSCLC cell lines via the ubiquitin-proteasome pathway.[124] SIRT3 can also mediated FOXO3a nuclear translocation that activates MnSOD and catalase expression.[124] Notably, SIRT1 knockdown cells can increase SIRT3 expression and cell survival and have relatively high resistance to H2O2 or etoposide treatment.[124] SIRT3 might be a therapeutic target for breast cancer, improving the effectiveness of cisplatin and tamoxifen treatments.[124] SIRT5 knockdown makes lung cancer cells more sensitive to drug (cisplatin, 5-fluorouracil or bleomycin) treatment.[124] SIRT5 depletion suppresses the expression of NRF2 and its downstream drug-resistance genes.[124]
Patients with high cytosol expression but low nuclear expression of SIRT6 can have poor clinical outcomes of lung cancer.[124] Furthermore, SIRT6 knockdown in NSCLC cell lines can improve paclitaxel sensitivity by reducing NF-κB and Beclin1 (autophagy mediator) levels.[124] Reduced SIRT6 expression mediates the augmentation of radiation-induced apoptosis via cAMP signaling in lung cancer cells.[124] A recent report revealed that SIRT7 depletion promotes gemcitabine-induced cell death.[124] Functioning as an oncogene, SIRT7 can be suppressed by miR-3666, which could increase NSCLC cell apoptosis.[124]
Thus, the aforementioned studies together have demonstrated tumor progression modulated by the SIRT1, SIRT3, and SIRT5-7, along with the tumor-suppressive effects of SIRT2 and SIRT4.[124] SIRT2 mediates the reactive oxygen species production and p27 levels, leading to lung cancer cell apoptosis and cell-cycle arrest.[124] SIRT2 overexpression increases NSCLC cells' sensitivity to cisplatin treatment.[124] Moreover, recent findings suggest that SIRT4 inhibits lung cancer progression through mitochondrial dynamics mediated by the ERK-Drp1 pathway.[124] As of 2020, one clinical trial (NCT02416739) is studying the combinatorial effects of the human sirtuin inhibitor (nicotinamide) and EGFR-TKI in NSCLC.[124] The discovery of specific SIRT regulation and EGFR-TKI treatment would help elucidate the roles of sirtuins in lung cancer development.[124] Although sirtuin clearly is critical in carcinogenesis, the crucial mechanisms by which the nicotine-mediated signaling or specific sirtuin pathways in different cell context lead to drug resistance require elucidation.[124]
Cell-membrane nAChRs implement upregulation of proliferative and survival genes.[124] Nicotine can promote oral precancerous growth through suppression of apoptosis by upregulating α7nAChR and peroxiredoxin.[124] α7nAChR-mediated cell protection, through JAK2/PI3K/AKT/signal transducer and activator of transcription 3(STAT3)/NF-κB activation, leads to Bcl-2 production.[124] Nicotine binds to nAChRs and stimulates secretion several factors including epidermal growth factor (EGF), VEGF, and neurotransmitters.[124] Nicotine/nAChRs mediates EGF secretion and subsequent EGFR signaling activation, thus contributing to antiapoptosis.[124] Nicotine and NNK also bind to β-ARs and promote survival signaling cascades.[124] Moreover, tissue-specific expression of α7β2, α3β2, α3β4, and α4β2 nAChRs located in the mitochondria outer membrane with anion channels that regulate the release of proapoptotic cytochrome c or reactive oxygen species production has been observed.[124] nAChR signaling in mitochondria is stimulated and engages PI3K/AKT kinases, similar to those activated by plasma membrane nAChRs.[124] Nicotine contributes to progression and erlotinib resistance in an NSCLC xenograft model through the nAChR-EGFR cooperation.[124] The nicotine-mediated α5nAChR/AKT signaling pathway prevents cisplatin-induced cancer cell apoptosis.[124] Blockade of α7nAChRs inhibited nicotine-induced tumor growth and vimentin expression in NSCLC through the RAS-RAF-MAPK kinase (MEK)-extracellular signal-regulated kinase (ERK) signaling pathway.[124] The nicotine and derivatives may mediate oncogenic signaling via nAChR, β-AR, and EGFR and combined with the effects of antiapoptosis in mitochondria that contribute to cancer progression.[124] The nicotine/nAChR signaling crosstalk with SIRT1/3/5-7 may contribute to cancer drug resistance.[124]
Risk assessment and mechanisms of carcinogenesis
Mechanism of action



When inhaled chemicals such as the carcinogenic chemicals found in tobacco smoke enter the body, they are capable of directly exerting their effects.[53] This can also occur indirectly by triggering persistent inflammation and metaplasia of the respiratory epithelium as a reaction to particulate matter.[53] In both situations, a large area of respiratory tissue is exposed to the causative agent, which in turn creates premalignant genetic alterations and the potential for cancer to eventually development.[53] Cancer is associated with the cumulative acquisition of genetic defects.[160] Mechanisms capable of inducing chronic inflammation and DNA damage are, therefore, involved in tumorigenesis.[160]
Emerging evidence of increased cancer risk
While the long-term health effects of e-cigarette use are still being studied, there is evidence to suggest that e-cigarette use may increase the risk of cancer as well as other diseases like cardiovascular and respiratory disease, due to the potential for harmful chemicals and flavorings in the aerosol.[19] Therefore, according to a 2023 review, it is important to exercise caution when using e-cigarettes and to consider alternative methods for smoking cessation.[19] According to a 2023 review, the safest course of action is to avoid using e-cigarettes altogether.[19]
Processes relating to the hallmarks of cancer
E-cigarette use promotes a variety of processes relating to the hallmarks of cancer and across the stages of disease progression.[20] While initial research has detected a decreased presence of carcinogen metabolites in the urine of e-cigarette users versus smokers, e-cigarette aerosol still contains carcinogens and may be capable of supporting tumorigenesis.[20] However, it is currently unclear whether e-cigarette aerosol predominantly promotes tumorigenesis directly, enhances primary tumor growth and survival, supports metastasis, or acts at all stages of cancer.[20]
Human microbiome and toxins
A 2020 study, with 119 participants (never smokers, tobacco smokers, e-cigarette users) showed that exposure to aerosol of e-liquid modulates the oral microbiome and elevates the abundance of oral pathobionts, induces gum inflammatory responses and makes epithelial cells more common to infection.[64]
In the oncology literature, there are reports indicating the possibility of a relationship between changes occurring within the human microbiome, inflammation and cancer development.[64] Bacteria can contribute to cancer processes by producing toxins, carcinogenic metabolites, and initiating chronic inflammation.[64]
The potential effects of next-generation products on oxidative stress are being debated.[159] There is conflicting data in the literature about the effects of next-generation products on oxidative stress-related carcinogenesis.[159] The aerosols produced from next-generation products (such as e-cigarettes) can result in oxidative stress.[159]
A 2017 study showed that e-cigarettes have a potent booster effect on phase I carcinogen bioactivation enzymes, including polycyclic aromatic hydrocarbon activators, and increase oxygen free radical production and DNA oxidation, which led to the creation of 8-Oxo-2'-deoxyguanosine.[159] A 2019 study showed that FVB/N mice exposed for 54 weeks to e-cigarette aerosol resulted in extensive DNA damage in the lungs, heart, and bladder mucosa and reduced DNA repair in the lungs.[159]
Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species and antioxidants,[160] in favor of oxidants.[159] In moderation, reactive oxygen species benefits the cell by regulating several cellular mechanisms that are protective against carcinogenesis.[160] Specifically, reactive oxygen species modulates antioxidant production, DNA repair, inflammatory responses, and cell growth and death.[160] When the amount of reactive oxygen species in the cell becomes excessive, the result is oxidative stress.[160] Oxidative stress may be caused by external cellular damage or by a failure of DNA repair systems.[160]
The protein NRF2 (also known as NFE2L2) is central to the regulation of antioxidant gene expression.[160] Under homeostatic conditions, NRF2 remains in the cytoplasm where it is bound to KEAP1.[160] KEAP1, together with CUL3 and RBX1, form the core ubiquitin ligase 3 complex.[160] When NRF2 is bound to the ubiquitin ligase 3 complex, it is degraded by the proteasome, which prevents it from accumulating in the cytoplasm.[160] When reactive oxygen species levels rise, the binding of NRF2 to KEAP1 is disrupted.[160] This allows NRF2 to escape protein degradation and to enter the nucleus.[160] Once inside the nucleus, NRF2 can initiate antioxidant transcription by forming a heterodimer with MAF proteins and binding to target gene sites.[160] The NRF2 signaling pathway regulates the transcription of over 500 genes and is an important mechanism of protection against oxidative stress.[160]
Risk of potential cancer initiation, development, and growth
There are many potential pathways by which e-cigarettes contribute to phenotypic changes known to be pro-oncogenic in nature.[20] E-cigarette use results in the production of reactive oxygen species that can contribute to DNA damage, and has been linked to double stranded DNA breaks and repair inhibition.[20] E-cigarette use is known to promote several pro-oncogenic phenomena that may support continuing tumor development after initiation.[20] Furthermore, e-cigarette use upregulates leukemia inhibitory factor which activates the MAPK and STAT3 pathways, both of which are well established oncogenic signaling pathways.[20]
E-cigarette use supports the growth and immune evasion of existing malignancies.[20] For instance, e-cigarette aerosol supports angiogenesis, which if occurring in a tumor, would facilitate the ongoing growth and survival of already established malignancies.[20] It is well established that platelet aggregation is enhanced by e-cigarette aerosol exposure; platelets also tend to "cloak" circulating tumor cells to protect them from immune detection and aid in circulating tumor cell adhesion to the endothelium.[20] Some e-cigarette aerosol-induced phenotypical changes are consistent with tumor-supporting processes and may indicate that e-cigarette use carries a risk of carcinogenesis; however, the severity of this risk remains unknown.[20] Given the relative novelty of vaping products, it is unknown how their long-term cancer risks compare to those of traditional tobacco or cannabis products.[161] As of 2023, the International Agency for Research on Cancer has not evaluated e-cigarettes for their potential risk for causing cancer.[162]
The development of cancer is caused by the accumulation of genetic damage.[53] Consequently, any mechanism that can cause DNA damage or DNA breaks can lead to the formation of cancer (carcinogenesis).[53] E-cigarette aerosols contain a variety of genotoxic substances (primarily as well as degradation by-products) that can cause single-strand DNA breaks, double-strand DNA breaks, and DNA mutations.[53] These include heavy metals, NNN, volatile organic compounds, polycyclic aromatic hydrocarbons, and reactive oxygen species.[53] These substances can also directly damage DNA.[53]
A 2018 study shows that e-cigarette aerosols with and without nicotine causes DNA breaks, though the genotoxicity was more pronounced in the e-cigarette aerosols that contained nicotine.[53] Moreover, the genotoxicity from the nicotine-infused e-cigarette aerosols was similar to that of traditional tobacco products.[53] These effects are corrected well with changes to the cell cycle (premature G1 and G2 arrest) and greater rates of apoptosis and necrosis, which led to causing cell death through both pathways.[53] As a result, extended use of e-cigarettes may cause escalating cycles of injury to DNA, accompanied by erroneous DNA repair, genetic abnormalities, and continuous further development of mutations, which may eventually lead to malignant transformation.[53]
DNA adduct formation caused by the by-products of heating e-liquid can also contribute to the development of cancer because their presence causes mutations.[160] Overall, smoking and vaping damage DNA via DNA adduct formation and thereby increase the likelihood of carcinogenesis.[160] DNA adducts are the product of covalent bonds of metabolically active carcinogens to DNA.[160] DNA damage is normally repaired by DNA polymerases.[160] However, in cases where a large amount of DNA damage is sustained, repair mechanisms cannot meet cellular demands, and mutational events can manifest, leading to cancer.[160] E-cigarette aerosols also induced single- and double-DNA-strand breaks. Both nicotine-containing and nicotine-free aerosols increase DNA breaks compared to controls; however, nicotine-containing aerosols show greater genotoxicity.[160] These findings were associated with altered cell cycle control and increased apoptosis. The chronic use of e-cigarettes could result in repeated DNA damage.[160] As DNA damage repair is error-prone, especially non-homologous end joining, it can allow for the accumulation of genomic aberrations.[160]
The smoking of traditional tobacco products or e-cigarette vaping have been described to cause inflammation and DNA mutations: Nicotine can be metabolized to the carcinogenic nitrosamine NNn and NNK.[160] E-cigarette usage may predispose users to the development of cancers just as traditional cigarette smoking does.[160] NNN is present in the saliva of e-cigarette users, in many cases showing similar NNN concentrations to those found in the saliva of traditional cigarette smokers.[160] Exposure to NNN can result directly from nicotine consumption, or it can form via the nitrosation of nicotine and nornicotine.[160] Reports indicate that NNN production likely occurs in the oral cavity, where nornicotine can interact with nitrite.[160] Other studies using rat models showed that NNN exposure resulted in the formation of tumors in the esophagus and the oral mucosa.[160]
The most direct evidence to date, as of 2023, linking vaping and cancer comes from an in vitro study in 2023 that demonstrates that flavored and unflavored e-cigarette aerosols are able to transform bronchial epithelial cells.[160] The alluring flavors and e-liquid alone are toxic, even in the absence of nicotine.[160] Tumor models have demonstrated that DNA damage and DNA repair inhibition induced by e-cigarette aerosols resulted in lung cancer and bladder precancer in rodent models.[160] This implies that the long-term health implications for a new generation of young people are grave, including addiction to nicotine and an increased risk for preventable cancers.[160]
Overall potential health risk
In addition to the potential cancer risk associated with e-cigarette use, there are other health concerns to consider.[19] E-cigarettes contain nicotine, which is addictive and can harm brain development in adolescents.[19] Nicotine can also raise blood pressure, increase heart rate, and constrict blood vessels, which can increase the risk of heart disease.[19] Many variables affect the levels of toxicants in the e-cigarette aerosol, including the design, the type of liquid, and user behavior.[163] Differences in the engineering and changes to the device itself by the user alters the nicotine uptake and the potential hazards.[88] Overall, while e-cigarettes may have very few benefits as a smoking cessation tool, the risks associated with their use, including the potential for cancer, should be carefully considered before use, according to a 2023 review.[19] Though, any substance breathed over an extended period of time may be harmful to the lungs, according to the European Respiratory Society.[19]
Etiology of role of nicotine in cancer initiation, development, and growth
Mounting evidence, over years, demonstrates that nicotine independently encourages the development of lung cancer via its interaction with nicotinic acetylcholine receptors.[164] For example, in 1989 it was discovered that nicotine stimulates the growth of lung cells.[164] Further, in 1990 research showed that nicotine hampers the demise of lung cancer cells.[164] The pathological angiogenesis of tumor growth and metastasis induced by nicotine was first reported by Heeschen et al in 2001.[165]
Related products
Traditional tobacco products

Tobacco use is the leading preventable cause of cancer and cancer deaths.[33] About 30% of all cancer deaths are caused by cigarette smoke.[166] Tobacco products can cause not only lung cancer — but also cancers of the mouth and throat, voice box, esophagus, stomach, kidney, pancreas, liver, bladder, cervix, colon and rectum, and a type of leukemia.[33] The risk of developing prostate cancer from smoking is minimal.[167] The risk of developing prostate cancer was higher in those that smoked tobacco and also ever used cannabis.[168]
Over the past 30 years leading up to 2019, more than 200 million deaths have been caused by tobacco smoking, and annual economic costs due to smoking tobacco use exceed US$1 trillion.[169] Smoking remains a defining challenge in global health.[169] Governments, and particularly ministers of health, face substantial obstacles ranging from population growth to pressure from the tobacco industry, to competing health and political priorities.[169]
Tobacco smoke contains approximately 7000 different chemicals, including nicotine, of which 93 chemicals of concern are proposed to produce direct or indirect harm through inhalation, ingestion, or absorption into the body.[23] Some of these toxicants are responsible for the onset of life-threatening medical conditions that affect the cardiovascular, respiratory, and digestive systems.[23]
Lung cancer is the leading cause of cancer death in the US and worldwide[170] and it remains the most common cause of being diagnosed with lung cancer.[171] Up to 90% of all lung cancer diagnoses are caused by classical cigarette use,[87] although lung cancer cases and mortality rates differ greatly worldwide.[172] This is due to risk factors such as individual classical cigarette use behaviors, the impact of environmental hazards, and genetic variability.[172] High concentrations of radon exposure combined with classical cigarette use is the second most common cause of developing lung cancer, according to a 2019 review.[173] In examining the combined effects of smoking and radon on lung cancer risk, both miner studies and residential radon studies showed that radon increases the risk of lung cancer for all persons: current smokers, ex-smokers, and lifelong non-smokers (never-smokers).[174] However, the absolute lung cancer risk due to radon for smokers and ex-smokers is higher than that of never-smokers.[174] Radon and its decay products are classified as group 1 carcinogens, according to International Agency for Research on Cancer.[175] Radon, a radioactive gas emanating from uranium,[176] is also a pulmonary carcinogen.[177] The risk of developing cancers associated with the use of tobacco products declines within a few years after quitting smoking.[178]
Herbal cigarettes
Herbal cigarettes, also referred as tobacco-free or nicotine-free cigarettes, are cigarettes that are free from tobacco and consist solely of a blend of herbs and/or other plant-based substances.[179] Meanwhile, there are other herbal cigarettes that do contain tobacco, which is complemented with other herbs to enhance flavor and the smoking experience.[179] Several companies promote herbal cigarettes as a safe substitute to smoking, but no solid evidence has been presented that these types of cigarettes have a favorable benefit on public health.[179] Herbal cigarettes are alternative smoking products that are often advertised as healthier than classical cigarettes and are especially popular in Asian markets.[180] The labelling of herbal cigarette unit packets in the European Union must mention that they are not less harmful for human health or more environmentally friendly.[180]
Although there is a limited understanding on the health effects of herbal cigarettes, a 2022 review suggests that herbal cigarettes are at least as harmful as classical cigarettes.[179] A 2009 study stated that Chinese herbal cigarettes are as carcinogenic and addictive as classical cigarettes.[85] Damiana, clove, peppermint, coltsfoot, lavender, and mugwort are among the primary plants used in herbal cigarettes.[179] The exact composition of herbal ingredients used in various herbal cigarettes and the way each ingredient was prepared is usually hidden from the public.[179] The majority of the toxic substances found in herbal cigarettes such as carbon monoxide, polyaromatics, aromatic amines, nicotine, and nitrosamines are also found in classical cigarettes, which may produce several undesirable health effects like causing cancer and heart disease.[179]
Heated tobacco products
Heated tobacco products generate both an aerosol[35] and smoke.[36] Prior to 2016, researchers at Philip Morris International stated that their IQOS product produces smoke[36] and the chemical evidence shows that the IQOS emissions fit the definition of both an aerosol and smoke.[37] Continual reheating of deposited tar in the IQOS device will occur with real-life use, likely leading to generation of even higher concentrations of harmful and potentially harmful compounds and particulate matter.[37] The IQOS product induces comparable respiratory epithelial toxicity to that of tobacco smoke and negatively affects cellular energetics and epithelial-to-mesenchymal transition, and causes oxidative stress.[53] These effects are correlated well with the possibility of causing malignant transformation.[53] In the heated tobacco product system, the tobacco plugs chars.[26] This charring increases when the device is not cleaned between the use of each heat stick.[26]
Their emissions contain levels of nicotine and carcinogens comparable to classical cigarettes.[38] The chemicals in the emissions of traditional cigarettes such as tar, nicotine, carbonyl compounds (including acetaldehyde, acrolein, and formaldehyde), and nitrosamines are also found in emissions of heated tobacco products.[181] These devices release formaldehyde cyanohydrin at 90 °C.[26] Even though heated tobacco products operate for a limited time, this is a concern, as it is highly toxic at low concentrations.[26]
Cannabis smoking
Smoked cannabis delivers THC and other cannabinoids to the body, but it also delivers harmful substances, including many of the same toxicants and carcinogens (cancer-causing chemicals) found in tobacco smoke, which are harmful to the lungs and cardiovascular system.[182] Cannabis smoke contains greater concentrations of polycyclic aromatic hydrocarbons and carcinogenic chemicals compared to that of tobacco smoke.[183] It also contains higher concentrations of tar than that of tobacco smoke.[183] Cannabis smoke is a potential respiratory tract carcinogen.[184]
The association between cannabis use and the development of cancer is not clear.[185] Chronic inflammatory and precancerous airway changes in a dose-dependent relationship are reported in cannabis users.[184] There is also reports of an increase in airway cancer in cannabis users.[184] Exposure to cannabis is associated with a twofold increase in the chance of developing lung cancer.[183] Limited evidence of an association between current, frequent, or chronic cannabis smoking and testicular cancer (non-seminoma-type) has been documented.[182] The evidence indicates a connection between consuming cannabis and an increased risk of cervical cancer.[185] The use of cannabis may also increase the probability of being diagnosed with breast cancer or laryngeal cancer.[185]
Research suggests that cannabis-only smokers are at lower risk of lung cancer than tobacco-only smokers.[184] However, some epidemiologic data does place an independent role of cannabis smoking in the development of lung cancer.[184] Despite epidemiological studies have struggled to definitively show a connection between cannabis smoking and cancer, based on the current data, a 2014 review explicitly advises against frequent, heavy use of cannabis (and possibly even moderate use).[186]
Smokeless tobacco products
28 carcinogens have been found in a wide variety of leading smokeless tobacco products.[note 9][188] The three categories of substances that were mainly found were non-volatile, alkaloid-derived tobacco-specific nitrosamines, nitrosoamino acids, and volatile nitrosamines.[188] Tobacco-specific nitrosamines were found to be the most plentiful and most carcinogenic among the recognized carcinogens in smokeless tobacco products.[188] Other detected carcinogenic chemicals include volatile aldehydes, polycyclic aromatic hydrocarbons, some lactones, urethane, and some metals.[188] The use of smokeless tobacco products is a known risk factor for causing oral, esophageal, and pancreatic cancers.[188]
Othar products
The potential for nicotine to facilitate cancer development and progression could also extend to other forms of nicotine delivery methods such as patches, nasal sprays, mouth sprays, inhalers, oral strips, chewing gum, lozenges, and microtabs.[52]
A 2022 study detected the existence of tobacco-specific nitrosamines in 26 of 44 nicotine pouch products.[189] The highest measured concentrations of NNN and NNK were 13 ng and 5.4 ng/pouch.[189] In addition to nicotine and tobacco-specific nitrosamines, toxic chromium and formaldehyde were detected in some of the nicotine pouch products.[189] Exposure to NNN is reported to be associated with promoting esophageal tumors.[189]
There is an association between the use of Swedish snus and a greater risk of esophagus, pancreas, stomach, and rectum cancers.[190] There is also an increased chance of death after being diagnosed with cancer.[190]
See also
Notes
- ↑ Nicotine’s carcinogenic toxicity is associated with the potential activation of oncogenes.[24] It also encourages tumorigenesis by inducing cell proliferation, migration, and invasion.[24] It may cause bladder, breast, cervical, colon, gastric, head and neck including nasopharynx, mtongue and oral cavity, lung, liver, and pancreatic cancers.[24]
- ↑ Acetaldehyde, benzene, cadmium, formaldehyde, isoprene, lead, nickel, nicotine, NNN, and toluene[39] are on the California's Proposition 65 list of chemicals known to the state to cause cancer, birth defects, or other reproductive harm.[40] There is evidence that the e-cigarette aerosol contains higher levels of other toxicants including heavy metals (such as tin and nickel) and silicate nanoparticles than that are present in classical cigarettes.[39]
- ↑ The user is referred to as a "vaper."[21]
- ↑ A "throat-hit" is a sensorial experience that is a form of airway irritation apparently resulting from nicotine use.[47]
- ↑ A 2016 report stated that, "many of the expert panelists who generated the '95% safer' claim were later shown to have connections to the tobacco industry and are established champions of e-cigarettes as 'harm reduction' devices; a strategy readily embraced by the tobacco industry."[60]
- ↑ The vast majority of these devices are being offered by the tobacco industry, whose track record of concealing and obfuscating data about tobacco safety is truly horrendous.[63] It took decades before it was appreciated that cigarette smoking caused lung cancer, and even longer until the incontrovertible evidence was widely accepted.[63]
- ↑ Several lines of evidence indicate that nicotine may contribute to the development of cancer.[102] Evidence from experimental in vitro studies on cell cultures, in vivo studies on rodents as well as studies on humans inclusive of epidemiological studies indicate that nicotine itself, independent of other tobacco constituents, may stimulate a number of effects of importance in cancer development.[102]
- ↑ E-cigarette aerosol generally contains fewer toxic chemicals than the deadly mix of 7,000 chemicals in smoke from classical cigarettes.[112]
- ↑ There is no safe level of smokeless tobacco use.[187] All tobacco products contain toxicants, and smokeless tobacco products contain cancer-causing chemicals.[187]
Bibliography
- National Center for Chronic Disease Prevention Health Promotion (US) Office on Smoking Health (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Surgeon General of the United States. pp. 1–943. PMID 24455788.
- "State Health Officer's Report on E-Cigarettes: A Community Health Threat" (PDF). California Tobacco Control Program. California Department of Public Health. January 2015. pp. 1–21.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - National Academies of Sciences, Engineering, and Medicine; et al. (National Academies of Sciences, Engineering, and Medicine and the Health and Medicine Division, Board on Population Health and Public Health Practice, and Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda of the National Academies of Sciences, Engineering, and Medicine) (31 March 2017). The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press. pp. 1–486. doi:10.17226/24625. ISBN 978-0-309-45307-3. PMID 28182367.
{{cite book}}: CS1 maint: multiple names: authors list (link) - McNeill, A; Brose, LS; Calder, R; Bauld, L; Robson, D (February 2018). "Evidence review of e-cigarettes and heated tobacco products 2018" (PDF). UK: Public Health England. pp. 1–243.
- National Academies of Sciences, Engineering, and Medicine; et al. (Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems) (23 January 2018). Stratton, Kathleen; Kwan, Leslie Y.; Eaton, David L. (eds.). Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press. pp. 1–774. doi:10.17226/24952. ISBN 978-0-309-46834-3. PMID 29894118.
{{cite book}}: CS1 maint: multiple names: authors list (link) Summary
References
- ↑ 1.0 1.1 Bandara, Nilanga Aki; Zhou, Xuan Randy; Alhamam, Abdullah; Black, Peter C.; St-Laurent, Marie-Pier (31 July 2023). "The genitourinary impacts of electronic cigarette use: a systematic review of the literature". World Journal of Urology. 41 (10): 2637–2646. doi:10.1007/s00345-023-04546-1. PMID 37524850.
- ↑ Wilson, Nick; Summers, Jennifer A.; Ait Ouakrim, Driss; Hoek, Janet; Edwards, Richard; Blakely, Tony (8 November 2021). "Improving on estimates of the potential relative harm to health from using modern ENDS (vaping) compared to tobacco smoking". BMC Public Health. 21 (1). doi:10.1186/s12889-021-12103-x. PMC 8577029. PMID 34749706.
 This article incorporates text by Nick Wilson,corresponding author1 Jennifer A. Summers, Driss Ait Ouakrim, Janet Hoek, Richard Edwards, and Tony Blakely2 available under the CC BY 4.0 license.
This article incorporates text by Nick Wilson,corresponding author1 Jennifer A. Summers, Driss Ait Ouakrim, Janet Hoek, Richard Edwards, and Tony Blakely2 available under the CC BY 4.0 license.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Marques, P; Piqueras, L; Sanz, MJ (18 May 2021). "An updated overview of e-cigarette impact on human health". Respiratory research. 22 (1): 151. doi:10.1186/s12931-021-01737-5. ISSN 1465-9921. PMC 8129966. PMID 34006276.
 This article incorporates text by Patrice Marques, Laura Piqueras, and Maria-Jesus Sanz available under the CC BY 4.0 license.
This article incorporates text by Patrice Marques, Laura Piqueras, and Maria-Jesus Sanz available under the CC BY 4.0 license.
- ↑ Bonner, Emily; Chang, Yvonne; Christie, Emerson; Colvin, Victoria; Cunningham, Brittany; Elson, Daniel; Ghetu, Christine; Huizenga, Juliana; Hutton, Sara J.; Kolluri, Siva K.; Maggio, Stephanie; Moran, Ian; Parker, Bethany; Rericha, Yvonne; Rivera, Brianna N.; Samon, Samantha; Schwichtenberg, Trever; Shankar, Prarthana; Simonich, Michael T.; Wilson, Lindsay B.; Tanguay, Robyn L. (September 2021). "The chemistry and toxicology of vaping". Pharmacology & Therapeutics. 225: 107837. doi:10.1016/j.pharmthera.2021.107837. PMC 8263470. PMID 33753133.
- ↑ McDonough, Samantha R.; Rahman, Irfan; Sundar, Isaac Kirubakaran (1 May 2021). "Recent updates on biomarkers of exposure and systemic toxicity in e-cigarette users and EVALI". American Journal of Physiology-Lung Cellular and Molecular Physiology. 320 (5): L661–L679. doi:10.1152/ajplung.00520.2020. PMC 8174828. PMID 33501893.
- ↑ Lynch, Jordan; Jin, Lexiao; Richardson, Andre; Conklin, Daniel J (September 2020). "Tobacco Smoke and Endothelial Dysfunction: Role of Aldehydes?". Current Hypertension Reports. 22 (9). doi:10.1007/s11906-020-01085-7. PMC 8108538. PMID 32857217.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Ruszkiewicz, Joanna A.; Zhang, Ziyan; Gonçalves, Filipe Marques; Tizabi, Yousef; Zelikoff, Judith T.; Aschner, Michael (2020). "Neurotoxicity of e-cigarettes". Food and Chemical Toxicology. 138: 111245. doi:10.1016/j.fct.2020.111245. ISSN 0278-6915. PMC 7089837. PMID 32145355.
- ↑ McGrath-Morrow, Sharon A.; Gorzkowski, Julie; Groner, Judith A.; Rule, Ana M.; Wilson, Karen; Tanski, Susanne E.; Collaco, Joseph M.; Klein, Jonathan D. (1 March 2020). "The Effects of Nicotine on Development". Pediatrics. 145 (3): e20191346. doi:10.1542/peds.2019-1346. PMC 7049940. PMID 32047098.
- ↑ Prochaska, Judith J.; Benowitz, Neal L. (11 October 2019). "Current advances in research in treatment and recovery: Nicotine addiction". Science Advances. 5 (10). doi:10.1126/sciadv.aay9763. PMC 6795520. PMID 31663029.
- ↑ Miliano, Cristina; Scott, E. Reilly; Murdaugh, Laura B.; Gnatowski, Emma R.; Faunce, Christine L.; Anderson, Megan S.; Reyes, Malissa M.; Gregus, Ann M.; Buczynski, Matthew W. (January 2020). "Modeling drug exposure in rodents using e-cigarettes and other electronic nicotine delivery systems". Journal of Neuroscience Methods. 330: 108458. doi:10.1016/j.jneumeth.2019.108458. PMC 7012754. PMID 31614162.
- ↑ Ghosh, Moyukh; Naderi, Sahar (June 2019). "Cannabis and Cardiovascular Disease". Current Atherosclerosis Reports. 21 (6). doi:10.1007/s11883-019-0783-9. PMID 30980200.
- ↑ Chadi, Nicholas; Hadland, Scott E.; Harris, Sion K. (January 2019). "Understanding the Implications of the "Vaping Epidemic" among Adolescents and Young Adults: A Call for Action". Substance Abuse. 40 (1): 7–10. doi:10.1080/08897077.2019.1580241. PMC 6583912. PMID 30883295.
- ↑ Strongin, Robert M. (12 June 2019). "E-Cigarette Chemistry and Analytical Detection". Annual Review of Analytical Chemistry. 12 (1): 23–39. doi:10.1146/annurev-anchem-061318-115329. PMC 6565477. PMID 30848928.
- ↑ Jenssen, Brian P.; Wilson, Karen M. (April 2019). "What is new in electronic-cigarettes research?". Current Opinion in Pediatrics. 31 (2): 262–266. doi:10.1097/MOP.0000000000000741. PMC 6644064. PMID 30762705.
- ↑ Unger, Michael; Unger, Darian W. (August 2018). "E-cigarettes/electronic nicotine delivery systems: a word of caution on health and new product development". Journal of Thoracic Disease. 10 (S22): S2588–S2592. doi:10.21037/jtd.2018.07.99. PMC 6178300. PMID 30345095.
- ↑ Ratajczak, Alexsandra; Feleszko, Wojciech; Smith, Danielle M.; Goniewicz, Maciej (3 July 2018). "How close are we to definitively identifying the respiratory health effects of e-cigarettes?". Expert Review of Respiratory Medicine. 12 (7): 549–556. doi:10.1080/17476348.2018.1483724. PMC 6310477. PMID 29856662.
- ↑ Haussmann, Hans-Juergen; Fariss, Marc W. (13 September 2016). "Comprehensive review of epidemiological and animal studies on the potential carcinogenic effects of nicotine per se". Critical Reviews in Toxicology. 46 (8): 701–734. doi:10.1080/10408444.2016.1182116. PMC 5020336. PMID 27278157.
- ↑ The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research 2017, p. 156.
- ↑ 19.000 19.001 19.002 19.003 19.004 19.005 19.006 19.007 19.008 19.009 19.010 19.011 19.012 19.013 19.014 19.015 19.016 19.017 19.018 19.019 19.020 19.021 19.022 19.023 19.024 19.025 19.026 19.027 19.028 19.029 19.030 19.031 19.032 19.033 19.034 19.035 19.036 19.037 19.038 19.039 19.040 19.041 19.042 19.043 19.044 19.045 19.046 19.047 19.048 19.049 19.050 19.051 19.052 19.053 19.054 19.055 19.056 19.057 19.058 19.059 19.060 19.061 19.062 19.063 19.064 19.065 19.066 19.067 19.068 19.069 19.070 19.071 19.072 19.073 19.074 19.075 19.076 19.077 19.078 19.079 19.080 19.081 19.082 19.083 19.084 19.085 19.086 19.087 19.088 19.089 19.090 19.091 19.092 19.093 19.094 19.095 19.096 19.097 19.098 19.099 19.100 19.101 19.102 19.103 19.104 19.105 19.106 19.107 19.108 19.109 19.110 19.111 19.112 19.113 19.114 19.115 19.116 19.117 19.118 19.119 19.120 19.121 19.122 19.123 19.124 19.125 19.126 19.127 19.128 19.129 19.130 Sahu, Rakesh; Shah, Kamal; Malviya, Rishabha; Paliwal, Deepika; Sagar, Sakshi; Singh, Sudarshan; Prajapati, Bhupendra G.; Bhattacharya, Sankha (14 November 2023). "E-Cigarettes and Associated Health Risks: An Update on Cancer Potential". Advances in Respiratory Medicine. 91 (6): 516–531. doi:10.3390/arm91060038. PMC 10660480. PMID 37987300.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Rakesh Sahu, Kamal Shah, Rishabha Malviya, Deepika Paliwal, Sakshi Sagar, Sudarshan Singh, Bhupendra G Prajapati, and Sankha Bhattacharya available under the CC BY 4.0 license.
This article incorporates text by Rakesh Sahu, Kamal Shah, Rishabha Malviya, Deepika Paliwal, Sakshi Sagar, Sudarshan Singh, Bhupendra G Prajapati, and Sankha Bhattacharya available under the CC BY 4.0 license.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 20.11 20.12 20.13 20.14 Snoderly, Hunter T.; Nurkiewicz, Timothy R.; Bowdridge, Elizabeth C.; Bennewitz, Margaret F. (18 November 2021). "E-Cigarette Use: Device Market, Study Design, and Emerging Evidence of Biological Consequences". International Journal of Molecular Sciences. MDPI AG. 22 (22): 12452. doi:10.3390/ijms222212452. ISSN 1422-0067. PMC 8619996. PMID 34830344.
 This article incorporates text by Hunter T. Snoderly,Timothy R. Nurkiewicz, Elizabeth C. Bowdridge, and Margaret F. Bennewitz available under the CC BY 4.0 license.
This article incorporates text by Hunter T. Snoderly,Timothy R. Nurkiewicz, Elizabeth C. Bowdridge, and Margaret F. Bennewitz available under the CC BY 4.0 license.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 Orellana-Barrios, Menfil A.; Payne, Drew; Mulkey, Zachary; Nugent, Kenneth (July 2015). "Electronic cigarettes-a narrative review for clinicians". The American Journal of Medicine. 128 (7): 674–681. doi:10.1016/j.amjmed.2015.01.033. ISSN 0002-9343. PMID 25731134.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 22.8 22.9 Zong, Huiqi; Hu, Zhekai; Li, Weina; Wang, Mina; Zhou, Qi; Li, Xiang; Liu, Hongxu (20 February 2024). "Electronic cigarettes and cardiovascular disease: epidemiological and biological links". Pflügers Archiv - European Journal of Physiology. doi:10.1007/s00424-024-02925-0. PMID 38376568.
 This article incorporates text by Huiqi Zong, Zhekai Hu, Weina Li, Mina Wang, Qi Zhou, Xiang Li, Hongxu Liu available under the CC BY 4.0 license.
This article incorporates text by Huiqi Zong, Zhekai Hu, Weina Li, Mina Wang, Qi Zhou, Xiang Li, Hongxu Liu available under the CC BY 4.0 license.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 23.9 Effah, Felix; Taiwo, Benjamin; Baines, Deborah; Bailey, Alexis; Marczylo, Tim (3 October 2022). "Pulmonary effects of e-liquid flavors: a systematic review". Journal of Toxicology and Environmental Health, Part B. 25 (7): 343–371. doi:10.1080/10937404.2022.2124563. PMC 9590402. PMID 36154615.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Felix Effah, Benjamin Taiwo, Deborah Baines, Alexis Bailey, and Tim Marczylo available under the CC BY 4.0 license.
This article incorporates text by Felix Effah, Benjamin Taiwo, Deborah Baines, Alexis Bailey, and Tim Marczylo available under the CC BY 4.0 license.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 Rao, Zihan; Xu, Yuqin; He, Zihan; Wang, Juan; Ji, Huanhong; Zhang, Zhongwei; Zhou, Jianming; Zhou, Tong; Wang, Huai (November 2023). "Carcinogenicity of nicotine and signal pathways in cancer progression: a review". Environmental Chemistry Letters. 22 (1): 239–272. doi:10.1007/s10311-023-01668-1.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 25.7 Chaturvedi, Pankaj; Mishra, Aseem; Datta, Sourav; Sinukumar, Snita; Joshi, Poonam; Garg, Apurva (2015). "Harmful effects of nicotine". Indian Journal of Medical and Paediatric Oncology. 36 (1): 24. doi:10.4103/0971-5851.151771. ISSN 0971-5851. PMC 4363846. PMID 25810571.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 Bravo-Gutiérrez, Omar Andrés; Falfán-Valencia, Ramcés; Ramírez-Venegas, Alejandra; Sansores, Raúl H.; Ponciano-Rodríguez, Guadalupe; Pérez-Rubio, Gloria (2021). "Lung Damage Caused by Heated Tobacco Products and Electronic Nicotine Delivery Systems: A Systematic Review". International Journal of Environmental Research and Public Health. 18 (8): 4079. doi:10.3390/ijerph18084079. ISSN 1660-4601. PMC 8070637. PMID 33924379.
 This article incorporates text by Omar Andrés Bravo-Gutiérrez, Ramcés Falfán-Valencia, Alejandra Ramírez-Venegas, Raúl H. Sansores, Guadalupe Ponciano-Rodríguez, and Gloria Pérez-Rubio1 available under the CC BY 4.0 license.
This article incorporates text by Omar Andrés Bravo-Gutiérrez, Ramcés Falfán-Valencia, Alejandra Ramírez-Venegas, Raúl H. Sansores, Guadalupe Ponciano-Rodríguez, and Gloria Pérez-Rubio1 available under the CC BY 4.0 license.
- ↑ 27.0 27.1 Jenssen, Brian P.; Walley, Susan C. (1 February 2019). "E-Cigarettes and Similar Devices". Pediatrics. 143 (2): e20183652. doi:10.1542/peds.2018-3652. ISSN 0031-4005. PMC 6644065. PMID 30835247.
- ↑ 28.0 28.1 28.2 28.3 Schraufnagel DE (March 2015). "Electronic Cigarettes: Vulnerability of Youth". Pediatric Allergy, Immunology, and Pulmonology. 28 (1): 2–6. doi:10.1089/ped.2015.0490. PMC 4359356. PMID 25830075.
- ↑ 29.0 29.1 29.2 England, Lucinda J.; Bunnell, Rebecca E.; Pechacek, Terry F.; Tong, Van T.; McAfee, Tim A. (August 2015). "Nicotine and the Developing Human". American Journal of Preventive Medicine. 49 (2): 286–93. doi:10.1016/j.amepre.2015.01.015. ISSN 0749-3797. PMC 4594223. PMID 25794473.
- ↑ 30.0 30.1 30.2 30.3 Li, Liqiao; Lin, Yan; Xia, Tian; Zhu, Yifang (2 April 2020). "Effects of Electronic Cigarettes on Indoor Air Quality and Health". Annual Review of Public Health. 41 (1): 363–380. doi:10.1146/annurev-publhealth-040119-094043. ISSN 0163-7525. PMC 7346849. PMID 31910714.
 This article incorporates text by Liqiao Li, Yan Lin, Tian Xia, and Yifang Zhu1 available under the CC BY 4.0 license.
This article incorporates text by Liqiao Li, Yan Lin, Tian Xia, and Yifang Zhu1 available under the CC BY 4.0 license.
- ↑ Samet, Jonathan M. (May 2013). "Tobacco Smoking". Thoracic Surgery Clinics. 23 (2): 103–112. doi:10.1016/j.thorsurg.2013.01.009. PMID 23566962.
- ↑ 32.0 32.1 32.2 32.3 32.4 32.5 32.6 Merecz-Sadowska, Anna; Sitarek, Przemyslaw; Zielinska-Blizniewska, Hanna; Malinowska, Katarzyna; Zajdel, Karolina; Zakonnik, Lukasz; Zajdel, Radoslaw (19 January 2020). "A Summary of In Vitro and In Vivo Studies Evaluating the Impact of E-Cigarette Exposure on Living Organisms and the Environment". International Journal of Molecular Sciences. 21 (2): 652. doi:10.3390/ijms21020652. ISSN 1422-0067. PMC 7013895. PMID 31963832.
 This article incorporates text by Anna Merecz-Sadowska, Przemyslaw Sitarek, Hanna Zielinska-Blizniewska, Katarzyna Malinowska, Karolina Zajdel, Lukasz Zakonnik, and Radoslaw Zajdel available under the CC BY 4.0 license.
This article incorporates text by Anna Merecz-Sadowska, Przemyslaw Sitarek, Hanna Zielinska-Blizniewska, Katarzyna Malinowska, Karolina Zajdel, Lukasz Zakonnik, and Radoslaw Zajdel available under the CC BY 4.0 license.
- ↑ 33.0 33.1 33.2 "Cancer and Tobacco Use". Centers for Disease Control and Prevention. 27 August 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 34.0 34.1 "Health Effects of Cigarette Smoking". Centers for Disease Control and Prevention. 29 October 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 35.0 35.1 "Heat-Not-Burn tobacco products information sheet". World Health Organization. May 2018.
- ↑ 36.0 36.1 36.2 36.3 Dautzenberg, B.; Dautzenberg, M.-D. (January 2019). "Le tabac chauffé : revue systématique de la littérature" [Systematic analysis of the scientific literature on heated tobacco]. Revue des Maladies Respiratoires (in français). 36 (1): 82–103. doi:10.1016/j.rmr.2018.10.010. ISSN 0761-8425. PMID 30429092.
- ↑ 37.0 37.1 37.2 37.3 37.4 Uguna, Clement N.; Snape, Colin E. (5 July 2022). "Should IQOS Emissions Be Considered as Smoke and Harmful to Health? A Review of the Chemical Evidence". ACS Omega. 7 (26): 22111–22124. doi:10.1021/acsomega.2c01527. PMC 9260752. PMID 35811880.
 This article incorporates text by Clement N. Uguna and Colin E. Snape available under the CC BY 4.0 license.
This article incorporates text by Clement N. Uguna and Colin E. Snape available under the CC BY 4.0 license.
- ↑ 38.0 38.1 Jenssen, Brian P.; Walley, Susan C.; McGrath-Morrow, Sharon A. (1 January 2018). "Heat-not-Burn Tobacco Products: Tobacco Industry Claims No Substitute for Science". Pediatrics. 141 (1): e20172383. doi:10.1542/peds.2017-2383. ISSN 0031-4005. PMID 29233936. S2CID 41704475.
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 State Health Officer's Report on E-Cigarettes: A Community Health Threat 2015, p. 6.
- ↑ 40.0 40.1 State Health Officer's Report on E-Cigarettes: A Community Health Threat 2015, p. 1.
- ↑ State Health Officer's Report on E-Cigarettes: A Community Health Threat 2015, p. 15.
- ↑ 42.0 42.1 42.2 42.3 42.4 42.5 Tang, Moon-shong; Lee, Hyun-Wook; Weng, Mao-wen; Wang, Hsiang-Tsui; Hu, Yu; Chen, Lung-Chi; Park, Sung-Hyun; Chan, Huei-wei; Xu, Jiheng; Wu, Xue-Ru; Wang, He; Yang, Rui; Galdane, Karen; Jackson, Kathryn; Chu, Annie; Halzack, Elizabeth (January 2022). "DNA damage, DNA repair and carcinogenicity: Tobacco smoke versus electronic cigarette aerosol". Mutation Research/Reviews in Mutation Research. 789: 108409. doi:10.1016/j.mrrev.2021.108409. PMC 9208310. PMID 35690412.
- ↑ 43.0 43.1 43.2 43.3 43.4 43.5 Sapru, Sakshi; Vardhan, Mridula; Li, Qianhao; Guo, Yuqi; Li, Xin; Saxena, Deepak (December 2020). "E-cigarettes use in the United States: reasons for use, perceptions, and effects on health". BMC Public Health. 20 (1). doi:10.1186/s12889-020-09572-x. PMC 7545933. PMID 33032554.
 This article incorporates text by Sakshi Sapru, Mridula Vardhan, Qianhao Li, Yuqi Guo, Xin Li, and Deepak Saxena available under the CC BY 4.0 license.
This article incorporates text by Sakshi Sapru, Mridula Vardhan, Qianhao Li, Yuqi Guo, Xin Li, and Deepak Saxena available under the CC BY 4.0 license.
- ↑ Pavelka, Margit; Roth, Jürgen (2010). "Alveoli: Gas Exchange and Host Defense". Functional Ultrastructure: Atlas of Tissue Biology and Pathology. Springer. pp. 248–249. ISBN 978-3-211-99390-3.
- ↑ 45.0 45.1 Henry, Travis S.; Kligerman, Seth J.; Raptis, Constantine A.; Mann, Howard; Sechrist, Jacob W.; Kanne, Jeffrey P. (2019). "Imaging Findings of Vaping-Associated Lung Injury". American Journal of Roentgenology: 1–8. doi:10.2214/AJR.19.22251. ISSN 0361-803X. PMID 31593518. S2CID 203985885.
- ↑ 46.0 46.1 Clapp, Phillip W.; Jaspers, Ilona (November 2017). "Electronic Cigarettes: Their Constituents and Potential Links to Asthma". Current Allergy and Asthma Reports. 17 (11): 79. doi:10.1007/s11882-017-0747-5. ISSN 1529-7322. PMC 5995565. PMID 28983782.
- ↑ Goldenson, Nicholas I.; Kirkpatrick, Matthew G.; Barrington-Trimis, Jessica L.; Pang, Raina D.; McBeth, Julia F.; Pentz, Mary Ann; Samet, Jonathan M.; Leventhal, Adam M. (2016). "Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology". Drug and Alcohol Dependence. 168: 176–180. doi:10.1016/j.drugalcdep.2016.09.014. ISSN 0376-8716. PMID 27676583.
- ↑ Virgili, Fabrizio; Nenna, Raffaella; Ben David, Shira; Mancino, Enrica; Di Mattia, Greta; Matera, Luigi; Petrarca, Laura; Midulla, Fabio (December 2022). "E-cigarettes and youth: an unresolved Public Health concern". Italian Journal of Pediatrics. 48 (1): 97. doi:10.1186/s13052-022-01286-7. PMC 9194784. PMID 35701844.
 This article incorporates text by Fabrizio Virgili, Raffaella Nenna, Shira Ben David, Enrica Mancino, Greta Di Mattia, Luigi Matera, Laura Petrarca, and Fabio Midulla available under the CC BY 4.0 license.
This article incorporates text by Fabrizio Virgili, Raffaella Nenna, Shira Ben David, Enrica Mancino, Greta Di Mattia, Luigi Matera, Laura Petrarca, and Fabio Midulla available under the CC BY 4.0 license.
- ↑ 49.0 49.1 49.2 Sachdeva, Jaspreet; Karunananthan, Anisha; Shi, Jianru; Dai, Wangde; Kleinman, Michael T; Herman, David; Kloner, Robert A (18 November 2023). "Flavoring Agents in E-cigarette Liquids: A Comprehensive Analysis of Multiple Health Risks". Cureus. doi:10.7759/cureus.48995. PMC 10726647. PMID 38111420.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Jaspreet Sachdeva, Anisha Karunananthan, Jianru Shi, Wangde Dai, Michael T Kleinman, David Herman, and Robert A Kloner available under the CC BY 4.0 license.
This article incorporates text by Jaspreet Sachdeva, Anisha Karunananthan, Jianru Shi, Wangde Dai, Michael T Kleinman, David Herman, and Robert A Kloner available under the CC BY 4.0 license.
- ↑ Montjean, Debbie; Godin Pagé, Marie-Hélène; Bélanger, Marie-Claire; Benkhalifa, Moncef; Miron, Pierre (18 March 2023). "An Overview of E-Cigarette Impact on Reproductive Health". Life. 13 (3): 827. doi:10.3390/life13030827. PMC 10053939. PMID 36983982.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Debbie Montjean, Marie-Hélène Godin Pagé, Marie-Claire Bélanger, Moncef Benkhalifa, and Pierre Miron available under the CC BY 4.0 license.
This article incorporates text by Debbie Montjean, Marie-Hélène Godin Pagé, Marie-Claire Bélanger, Moncef Benkhalifa, and Pierre Miron available under the CC BY 4.0 license.
- ↑ "Must Know Facts About E-Cigarettes | Vaping 101 | Vaping Prevention Resources". United States Food and Drug Administration. United States Department of Health and Human Services. 2023.
- ↑ 52.00 52.01 52.02 52.03 52.04 52.05 52.06 52.07 52.08 52.09 52.10 52.11 52.12 52.13 Mravec, Boris; Tibensky, Miroslav; Horvathova, Lubica; Babal, Pavel (2020). "E-Cigarettes and Cancer Risk". Cancer Prevention Research. 13 (2): 137–144. doi:10.1158/1940-6207.CAPR-19-0346. ISSN 1940-6207. PMID 31619443.
- ↑ 53.00 53.01 53.02 53.03 53.04 53.05 53.06 53.07 53.08 53.09 53.10 53.11 53.12 53.13 53.14 53.15 53.16 53.17 53.18 Bracken-Clarke, Dara; Kapoor, Dhruv; Baird, Anne Marie; Buchanan, Paul James; Gately, Kathy; Cuffe, Sinead; Finn, Stephen P. (2021). "Vaping and lung cancer – A review of current data and recommendations". Lung Cancer. 153: 11–20. doi:10.1016/j.lungcan.2020.12.030. ISSN 0169-5002. PMID 33429159.
- ↑ Lai, Lo; Qiu, Hongyu (April 2021). "Biological Toxicity of the Compositions in Electronic-Cigarette on Cardiovascular System". Journal of Cardiovascular Translational Research. 14 (2): 371–376. doi:10.1007/s12265-020-10060-1. ISSN 1937-5387. PMC 7855637. PMID 32748205.
- ↑ 55.0 55.1 55.2 Walley, Susan C.; Wilson, Karen M.; Winickoff, Jonathan P.; Groner, Judith (June 2019). "A Public Health Crisis: Electronic Cigarettes, Vape, and JUUL". Pediatrics. 143 (6): e20182741. doi:10.1542/peds.2018-2741. ISSN 0031-4005. PMID 31122947.
- ↑ 56.0 56.1 56.2 Raj, A. Thirumal; Sujatha, Govindarajan; Muruganandhan, Jayanandan; Kumar, S. Satish; Bharkavi, SK Indu; Varadarajan, Saranya; Patil, Shankargouda; Awan, Kamran Habib (April 2020). "Reviewing the oral carcinogenic potential of E-cigarettes using the Bradford Hill criteria of causation". Translational Cancer Research. 9 (4): 3142–3152. doi:10.21037/tcr.2020.01.23. PMC 8798817. PMID 35117678.
- ↑ 57.0 57.1 57.2 Binns, Colin; Lee, Mi Kyung; Low, Wah Yun (May 2018). "Children and E-Cigarettes: A New Threat to Health". Asia Pacific Journal of Public Health. 30 (4): 315–320. doi:10.1177/1010539518783808. PMID 29978722.
- ↑ Amin, Samia; Dunn, Adam G.; Laranjo, Liliana (January 2020). "Social Influence in the Uptake and Use of Electronic Cigarettes: A Systematic Review". American Journal of Preventive Medicine. 58 (1): 129–141. doi:10.1016/j.amepre.2019.08.023. PMID 31761515.
- ↑ Jenssen, Brian P.; Boykan, Rachel (20 February 2019). "Electronic Cigarettes and Youth in the United States: A Call to Action (at the Local, National and Global Levels)". Children. 6 (2): 30. doi:10.3390/children6020030. ISSN 2227-9067. PMC 6406299. PMID 30791645.
 This article incorporates text by Brian P. Jenssen and Rachel Boykan available under the CC BY 4.0 license.
This article incorporates text by Brian P. Jenssen and Rachel Boykan available under the CC BY 4.0 license.
- ↑ Couch, Elizabeth T.; Chaffee, Benjamin W.; Gansky, Stuart A.; Walsh, Margaret M. (July 2016). "The changing tobacco landscape". The Journal of the American Dental Association. 147 (7): 561–569. doi:10.1016/j.adaj.2016.01.008. ISSN 0002-8177. PMC 4925234. PMID 26988178.
- ↑ Lødrup Carlsen, Karin C.; Skjerven, Håvard O.; Carlsen, Kai-Håkon (September 2018). "The toxicity of E-cigarettes and children's respiratory health". Paediatric Respiratory Reviews. 28: 63–67. doi:10.1016/j.prrv.2018.01.002. PMID 29580719.
- ↑ 62.0 62.1 62.2 McCaughey, Conor James; Murphy, Greg; Jones, Jennifer; Mirza, Kaumal Baig; Hensey, Mark (December 2023). "Safety and efficacy of e-cigarettes in those with atherosclerotic disease: a review". Open Heart. 10 (2): e002341. doi:10.1136/openhrt-2023-002341. PMC 10711928. PMID 38065586.
{{cite journal}}: Check|pmc=value (help) - ↑ 63.0 63.1 63.2 Bush, Andrew; Lintowska, Agnieszka; Mazur, Artur; Hadjipanayis, Adamos; Grossman, Zacchi; del Torso, Stefano; Michaud, Pierre-André; Doan, Svitlana; Romankevych, Ivanna; Slaats, Monique; Utkus, Algirdas; Dembiński, Łukasz; Slobodanac, Marija; Valiulis, Arunas (4 October 2021). "E-Cigarettes as a Growing Threat for Children and Adolescents: Position Statement From the European Academy of Paediatrics". Frontiers in Pediatrics. 9. doi:10.3389/fped.2021.698613. PMC 8562300. PMID 34737999.
 This article incorporates text by Andrew Bush, Agnieszka Lintowska, Artur Mazur, Adamos Hadjipanayis, Zacchi Grossman, Stefano del Torso, Pierre-André Michaud, Svitlana Doan, Ivanna Romankevych, Monique Slaats, Algirdas Utkus, Łukasz Dembiński, Marija Slobodanac, and Arunas Valiulis available under the CC BY 4.0 license.
This article incorporates text by Andrew Bush, Agnieszka Lintowska, Artur Mazur, Adamos Hadjipanayis, Zacchi Grossman, Stefano del Torso, Pierre-André Michaud, Svitlana Doan, Ivanna Romankevych, Monique Slaats, Algirdas Utkus, Łukasz Dembiński, Marija Slobodanac, and Arunas Valiulis available under the CC BY 4.0 license.
- ↑ 64.0 64.1 64.2 64.3 64.4 64.5 64.6 Szukalska, Marta; Szyfter, Krzysztof; Florek, Ewa; Rodrigo, Juan P.; Rinaldo, Alessandra; Mäkitie, Antti A.; Strojan, Primož; Takes, Robert P.; Suárez, Carlos; Saba, Nabil F.; Braakhuis, Boudewijn J.M.; Ferlito, Alfio (5 November 2020). "Electronic Cigarettes and Head and Neck Cancer Risk—Current State of Art". Cancers. 12 (11): 3274. doi:10.3390/cancers12113274. PMC 7694366. PMID 33167393.
 This article incorporates text by Marta Szukalska, Krzysztof Szyfter, Ewa Florek, Juan P. Rodrigo, Alessandra Rinaldo, Antti A. Mäkitie, Primož Strojan, Robert P. Takes, Carlos Suárez, Nabil F. Saba, Boudewijn J.M. Braakhuis, and Alfio Ferlito available under the CC BY 4.0 license.
This article incorporates text by Marta Szukalska, Krzysztof Szyfter, Ewa Florek, Juan P. Rodrigo, Alessandra Rinaldo, Antti A. Mäkitie, Primož Strojan, Robert P. Takes, Carlos Suárez, Nabil F. Saba, Boudewijn J.M. Braakhuis, and Alfio Ferlito available under the CC BY 4.0 license.
- ↑ 65.0 65.1 Bekki, Kanae; Uchiyama, Shigehisa; Ohta, Kazushi; Inaba, Yohei; Nakagome, Hideki; Kunugita, Naoki (2014). "Carbonyl Compounds Generated from Electronic Cigarettes". International Journal of Environmental Research and Public Health. 11 (11): 11192–11200. doi:10.3390/ijerph111111192. ISSN 1660-4601. PMC 4245608. PMID 25353061.
- ↑ 66.0 66.1 66.2 66.3 66.4 Hikisz, Pawel; Jacenik, Damian (11 March 2023). "The Tobacco Smoke Component, Acrolein, as a Major Culprit in Lung Diseases and Respiratory Cancers: Molecular Mechanisms of Acrolein Cytotoxic Activity". Cells. 12 (6): 879. doi:10.3390/cells12060879. PMC 10047238. PMID 36980220.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Pawel Hikisz1 and Damian Jacenik available under the CC BY 4.0 license.
This article incorporates text by Pawel Hikisz1 and Damian Jacenik available under the CC BY 4.0 license.
- ↑ Elikkottil, J; Gupta, P; Gupta, K (November 2009). "The analgesic potential of cannabinoids". Journal of opioid management. 5 (6): 341–57. PMC 3728280. PMID 20073408.
- ↑ 68.0 68.1 68.2 de Groot, Patricia M.; Wu, Carol C.; Carter, Brett W.; Munden, Reginald F. (June 2018). "The epidemiology of lung cancer". Translational Lung Cancer Research. 7 (3): 220–233. doi:10.21037/tlcr.2018.05.06. PMC 6037963. PMID 30050761.
- ↑ 69.0 69.1 Veerappa, Avinash; Pendyala, Gurudutt; Guda, Chittibabu (November 2021). "A systems omics-based approach to decode substance use disorders and neuroadaptations". Neuroscience & Biobehavioral Reviews. 130: 61–80. doi:10.1016/j.neubiorev.2021.08.016. PMC 8511293. PMID 34411560.
- ↑ 70.0 70.1 Fordjour, Eric; Manful, Charles F.; Sey, Albert A.; Javed, Rabia; Pham, Thu Huong; Thomas, Raymond; Cheema, Mumtaz (15 June 2023). "Cannabis: a multifaceted plant with endless potentials". Frontiers in Pharmacology. 14. doi:10.3389/fphar.2023.1200269. PMC 10308385. PMID 37397476.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Eric Fordjour, Charles F. Manful, Albert A. Sey, Rabia Javed, Thu Huong Pham, Raymond Thomas, and Mumtaz Cheema available under the CC BY 4.0 license.
This article incorporates text by Eric Fordjour, Charles F. Manful, Albert A. Sey, Rabia Javed, Thu Huong Pham, Raymond Thomas, and Mumtaz Cheema available under the CC BY 4.0 license.
- ↑ 71.0 71.1 71.2 71.3 Gordon, Adam J.; Conley, James W.; Gordon, Joanne M. (December 2013). "Medical Consequences of Marijuana Use: A Review of Current Literature". Current Psychiatry Reports. 15 (12). doi:10.1007/s11920-013-0419-7. PMID 24234874.
- ↑ The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research 2017, p. 156, Cancer.
- ↑ Agolli, Arjola; Agolli, Olsi; Chowdhury, Selia; Shet, Vallabh; Benitez, Johanna S. Canenguez; Bheemisetty, Niharika; Waleed, Madeeha Subhan (30 June 2022). "Increased cannabis use in pregnant women during COVID-19 pandemic". Discoveries. 10 (2): e148. doi:10.15190/d.2022.7. PMC 9748245. PMID 36530177.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Arjola Agolli, Olsi Agolli, Selia Chowdhury, Vallabh Shet, Johanna S. Canenguez Benitez, Niharika Bheemisetty, and Madeeha Subhan Waleed available under the CC BY 4.0 license.
This article incorporates text by Arjola Agolli, Olsi Agolli, Selia Chowdhury, Vallabh Shet, Johanna S. Canenguez Benitez, Niharika Bheemisetty, and Madeeha Subhan Waleed available under the CC BY 4.0 license.
- ↑ 74.0 74.1 74.2 McAlinden, Kielan Darcy; Eapen, Mathew Suji; Lu, Wenying; Sharma, Pawan; Sohal, Sukhwinder Singh (2020). "The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease". American Journal of Physiology-Lung Cellular and Molecular Physiology. 319 (4): L585–L595. doi:10.1152/ajplung.00160.2020. ISSN 1040-0605. PMID 32726146.
- ↑ 75.0 75.1 75.2 75.3 75.4 75.5 Shehata, Shaimaa A.; Toraih, Eman A.; Ismail, Ezzat A.; Hagras, Abeer M.; Elmorsy, Ekramy; Fawzy, Manal S. (12 September 2023). "Vaping, Environmental Toxicants Exposure, and Lung Cancer Risk". Cancers. 15 (18): 4525. doi:10.3390/cancers15184525. PMC 10526315. PMID 37760496.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Shaimaa A Shehata, Eman A Toraih, Ezzat A Ismail, Abeer M Hagras, Ekramy Elmorsy, and Manal S Fawzy available under the CC BY 4.0 license.
This article incorporates text by Shaimaa A Shehata, Eman A Toraih, Ezzat A Ismail, Abeer M Hagras, Ekramy Elmorsy, and Manal S Fawzy available under the CC BY 4.0 license.
- ↑ 76.0 76.1 76.2 76.3 76.4 76.5 76.6 Gurney, J.; Shaw, C.; Stanley, J.; Signal, V.; Sarfati, D. (December 2015). "Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis". BMC Cancer. 15 (1). doi:10.1186/s12885-015-1905-6. PMC 4642772. PMID 26560314.
 This article incorporates text by J. Gurney, C. Shaw, J. Stanley, V. Signal, and D. Sarfati available under the CC BY 4.0 license.
This article incorporates text by J. Gurney, C. Shaw, J. Stanley, V. Signal, and D. Sarfati available under the CC BY 4.0 license.
- ↑ 77.0 77.1 Tøttenborg, SS; Holm, AL; Wibholm, NC; Lange, P (1 September 2014). "E-cigaretten indeholder også skadelige stoffer" [E-cigarettes also contain detrimental chemicals]. Ugeskrift for laeger (in Danish). 176 (36). PMID 25293843.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ "Summary of Results: Laboratory Analysis of Electronic Cigarettes Conducted By FDA". United States Food and Drug Administration. 22 April 2014. Archived from the original on 29 June 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 79.0 79.1 Rumgay, Harriet; Murphy, Neil; Ferrari, Pietro; Soerjomataram, Isabelle (11 September 2021). "Alcohol and Cancer: Epidemiology and Biological Mechanisms". Nutrients. 13 (9): 3173. doi:10.3390/nu13093173. PMC 8470184. PMID 34579050.
 This article incorporates text by Harriet Rumgay, Neil Murphy, Pietro Ferrari, and Isabelle Soerjomataram available under the CC BY 4.0 license.
This article incorporates text by Harriet Rumgay, Neil Murphy, Pietro Ferrari, and Isabelle Soerjomataram available under the CC BY 4.0 license.
- ↑ 80.0 80.1 Public Health Consequences of E-Cigarettes 2018, p. 172, Toxicology of E-Cigarette Constituents.
- ↑ Dinakar, Chitra; Longo, Dan L.; O'Connor, George T. (6 October 2016). "The Health Effects of Electronic Cigarettes". New England Journal of Medicine. 375 (14): 1372–1381. doi:10.1056/NEJMra1502466. ISSN 0028-4793. PMID 27705269.
- ↑ 82.0 82.1 Stefaniak, Aleksandr B.; LeBouf, Ryan F.; Ranpara, Anand C.; Leonard, Stephen S. (2021). "Toxicology of flavoring- and cannabis-containing e-liquids used in electronic delivery systems". Pharmacology & Therapeutics. 224: 107838. doi:10.1016/j.pharmthera.2021.107838. ISSN 0163-7258. PMC 8251682. PMID 33746051.
- ↑ Public Health Consequences of E-Cigarettes 2018, p. 156, Toxicology of E-Cigarette Constituents.
- ↑ Public Health Consequences of E-Cigarettes 2018, p. 167, Toxicology of E-Cigarette Constituents.
- ↑ 85.0 85.1 85.2 85.3 85.4 Kleinstreuer, Clement; Feng, Yu (23 September 2013). "Lung Deposition Analyses of Inhaled Toxic Aerosols in Conventional and Less Harmful Cigarette Smoke: A Review". International Journal of Environmental Research and Public Health. 10 (9): 4454–4485. doi:10.3390/ijerph10094454. PMC 3799535. PMID 24065038.
 This article incorporates text by Clement Kleinstreuer and Yu Feng available under the CC BY 3.0 license.
This article incorporates text by Clement Kleinstreuer and Yu Feng available under the CC BY 3.0 license.
- ↑ Bautista, Malia; Mogul, Allison S.; Fowler, Christie D. (14 August 2023). "Beyond the label: current evidence and future directions for the interrelationship between electronic cigarettes and mental health". Frontiers in Psychiatry. 14. doi:10.3389/fpsyt.2023.1134079. PMC 10460914. PMID 37645635.
{{cite journal}}: Check|pmc=value (help) - ↑ 87.0 87.1 87.2 Chaitanya Thandra, Krishna; Barsouk, Adam; Saginala, Kalyan; Sukumar Aluru, John; Barsouk, Alexander (February 2021). "Epidemiology of lung cancer". Współczesna Onkologia. 25 (1): 45–52. doi:10.5114/wo.2021.103829. PMC 8063897. PMID 33911981.
- ↑ 88.0 88.1 88.2 Brown, Christopher J; Cheng, James M (May 2014). "Electronic cigarettes: product characterisation and design considerations". Tobacco Control. 23 (suppl 2): ii4–ii10. doi:10.1136/tobaccocontrol-2013-051476. ISSN 0964-4563. PMC 3995271. PMID 24732162.
- ↑ 89.0 89.1 Kaur, Gagandeep; Pinkston, Rakeysha; Mclemore, Benathel; Dorsey, Waneene C.; Batra, Sanjay (2018). "Immunological and toxicological risk assessment of e-cigarettes". European Respiratory Review. 27 (147): 170119. doi:10.1183/16000617.0119-2017. ISSN 0905-9180. PMC 9489161. PMID 29491036.
- ↑ 90.0 90.1 Public Health Consequences of E-Cigarettes 2018, p. 200, Metals.
- ↑ England, Lucinda (1 November 2015). "Important Considerations for Providers Regarding the Use of Electronic Cigarettes". International Journal of Respiratory and Pulmonary Medicine. 2 (4). doi:10.23937/2378-3516/1410035. ISSN 2378-3516.
 This article incorporates text by Lucinda England, Joseph G. Lisko, and R. Steven Pappas available under the CC BY 4.0 license.
This article incorporates text by Lucinda England, Joseph G. Lisko, and R. Steven Pappas available under the CC BY 4.0 license.
- ↑ 92.0 92.1 Traboulsi, Hussein; Cherian, Mathew; Abou Rjeili, Mira; Preteroti, Matthew; Bourbeau, Jean; Smith, Benjamin M.; Eidelman, David H.; Baglole, Carolyn J. (15 May 2020). "Inhalation Toxicology of Vaping Products and Implications for Pulmonary Health". International Journal of Molecular Sciences. 21 (10): 3495. doi:10.3390/ijms21103495. ISSN 1422-0067. PMC 7278963. PMID 32429092.
 This article incorporates text by Hussein Traboulsi, Mathew Cherian, Mira Abou Rjeili, Matthew Preteroti, Jean Bourbeau, Benjamin M. Smith, David H. Eidelman, and Carolyn J. Baglole available under the CC BY 4.0 license.
This article incorporates text by Hussein Traboulsi, Mathew Cherian, Mira Abou Rjeili, Matthew Preteroti, Jean Bourbeau, Benjamin M. Smith, David H. Eidelman, and Carolyn J. Baglole available under the CC BY 4.0 license.
- ↑ 93.0 93.1 Benowitz, Neal L.; Fraiman, Joseph B. (2017). "Cardiovascular effects of electronic cigarettes". Nature Reviews Cardiology. 14 (8): 447–456. doi:10.1038/nrcardio.2017.36. ISSN 1759-5002. PMC 5519136. PMID 28332500.
- ↑ 94.0 94.1 94.2 Schick, Suzaynn F.; Blount, Benjamin C; Jacob, Peyton; Saliba, Najat A; Bernert, John T; El Hellani, Ahmad; Jatlow, Peter; Pappas, R Steve; Wang, Lanqing; Foulds, Jonathan; Ghosh, Arunava; Hecht, Stephen S; Gomez, John C; Martin, Jessica R; Mesaros, Clementina; Srivastava, Sanjay; St. Helen, Gideon; Tarran, Robert; Lorkiewicz, Pawel K; Blair, Ian A; Kimmel, Heather L; Doerschuk, Claire M.; Benowitz, Neal L; Bhatnagar, Aruni (2017). "Biomarkers of Exposure to New and Emerging Tobacco and Nicotine Delivery Products". American Journal of Physiology. Lung Cellular and Molecular Physiology. 313 (3): L425–L452. doi:10.1152/ajplung.00343.2016. ISSN 1040-0605. PMC 5626373. PMID 28522563.
- ↑ Livingston, Catherine J.; Freeman, Randall J.; Costales, Victoria C.; Westhoff, John L.; Caplan, Lee S.; Sherin, Kevin M.; Niebuhr, David W. (2019). "Electronic Nicotine Delivery Systems or E-cigarettes: American College of Preventive Medicine's Practice Statement". American Journal of Preventive Medicine. 56 (1): 167–178. doi:10.1016/j.amepre.2018.09.010. ISSN 0749-3797. PMID 30573147.
- ↑ Bhalerao, Aditya; Sivandzade, Farzane; Archie, Sabrina Rahman; Cucullo, Luca (2019). "Public Health Policies on E-Cigarettes". Current Cardiology Reports. 21 (10). doi:10.1007/s11886-019-1204-y. ISSN 1523-3782. PMC 6713696. PMID 31463564.
 This article incorporates text by Aditya Bhalerao, Farzane Sivandzade, Sabrina Rahman Archie, and Luca Cucullo available under the CC BY 4.0 license.
This article incorporates text by Aditya Bhalerao, Farzane Sivandzade, Sabrina Rahman Archie, and Luca Cucullo available under the CC BY 4.0 license.
- ↑ 97.0 97.1 97.2 97.3 97.4 97.5 97.6 Taylor, Eve; Simonavičius, Erikas; McNeill, Ann; Brose, Leonie S; East, Katherine; Marczylo, Tim; Robson, Debbie (22 February 2024). "Exposure to Tobacco-Specific Nitrosamines Among People Who Vape, Smoke, or do Neither: A Systematic Review and Meta-Analysis". Nicotine and Tobacco Research. 26 (3): 257–269. doi:10.1093/ntr/ntad156. PMC 10882431. PMID 37619211.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Eve Taylor, Erikas Simonavičius, Ann McNeill, Leonie S Brose, Katherine East, Tim Marczylo, and Debbie Robson available under the CC BY 4.0 license.
This article incorporates text by Eve Taylor, Erikas Simonavičius, Ann McNeill, Leonie S Brose, Katherine East, Tim Marczylo, and Debbie Robson available under the CC BY 4.0 license.
- ↑ Cooke, John; Bitterman, Haim (January 2004). "Nicotine and angiogenesis: a new paradigm for tobacco‐related diseases". Annals of Medicine. 36 (1): 33–40. doi:10.1080/07853890310017576. PMID 15000345.
- ↑ Schaal, Courtney; Chellappan, Srikumar P. (16 January 2014). "Nicotine-Mediated Cell Proliferation and Tumor Progression in Smoking-Related Cancers". Molecular Cancer Research. 12 (1): 14–23. doi:10.1158/1541-7786.MCR-13-0541. PMC 3915512. PMID 24398389.
- ↑ 100.0 100.1 Yalcin, Emine; de la Monte, Suzanne (March 2016). "Tobacco nitrosamines as culprits in disease: mechanisms reviewed". Journal of Physiology and Biochemistry. 72 (1): 107–120. doi:10.1007/s13105-016-0465-9. PMC 4868960. PMID 26767836.
- ↑ "A nicotine-free vape is not a worry-free vape" (PDF). United States Food and Drug Administration. United States Department of Health and Human Services. 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 102.0 102.1 102.2 102.3 102.4 102.5 102.6 Sanner, Tore; Grimsrud, Tom K. (31 August 2015). "Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment – A Review". Frontiers in Oncology. 5: 196. doi:10.3389/fonc.2015.00196. ISSN 2234-943X. PMC 4553893. PMID 26380225.
 This article incorporates text by Tore Sanner, and Tom K. Grimsrud available under the CC BY 4.0 license.
This article incorporates text by Tore Sanner, and Tom K. Grimsrud available under the CC BY 4.0 license.
- ↑ Grando, Sergei A. (June 2014). "Connections of nicotine to cancer". Nature Reviews Cancer. 14 (6): 419–429. doi:10.1038/nrc3725. PMID 24827506.
- ↑ The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General 2014, p. 115.
- ↑ The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General 2014, p. 116.
- ↑ Schuller, Hildegard M. (March 2009). "Is cancer triggered by altered signalling of nicotinic acetylcholine receptors?". Nature Reviews Cancer. 9 (3): 195–205. doi:10.1038/nrc2590. PMID 19194381.
- ↑ 107.0 107.1 107.2 107.3 107.4 Shin, Vivian Y.; Cho, Chi-Hin (April 2005). "Nicotine and gastric cancer". Alcohol. 35 (3): 259–264. doi:10.1016/j.alcohol.2005.04.007. PMID 16054988.
- ↑ 108.0 108.1 108.2 Singh, Jasjot; Luquet, Emilie; Smith, DavidP.T.; Potgieter, HermanJ.; Ragazzon, Patricia (2016). "Toxicological and analytical assessment of e-cigarette refill components on airway epithelia" (PDF). Science Progress. 99 (4): 351–398. doi:10.3184/003685016X14773090197706. ISSN 0036-8504. PMID 28742478.
- ↑ 109.00 109.01 109.02 109.03 109.04 109.05 109.06 109.07 109.08 109.09 109.10 109.11 109.12 109.13 109.14 Esteban-Lopez, Maria; Perry, Marissa D.; Garbinski, Luis D.; Manevski, Marko; Andre, Mickensone; Ceyhan, Yasemin; Caobi, Allen; Paul, Patience; Lau, Lee Seng; Ramelow, Julian; Owens, Florida; Souchak, Joseph; Ales, Evan; El-Hage, Nazira (2022). "Health effects and known pathology associated with the use of E-cigarettes". Toxicology Reports. 9: 1357–1368. doi:10.1016/j.toxrep.2022.06.006. PMC 9764206. PMID 36561957.
{{cite journal}}: Check|pmc=value (help) - ↑ Bruin, Jennifer E.; Gerstein, Hertzel C.; Holloway, Alison C. (August 2010). "Long-Term Consequences of Fetal and Neonatal Nicotine Exposure: A Critical Review". Toxicological Sciences. 116 (2): 364–374. doi:10.1093/toxsci/kfq103. PMC 2905398. PMID 20363831.
- ↑ Riwu Bara, Roy Pefi; McCausland, Kahlia; Swanson, Maurice; Scott, Lucy; Jancey, Jonine (February 2023). ""They're sleek, stylish and sexy:" selling e-cigarettes online". Australian and New Zealand Journal of Public Health. 47 (1): 100013. doi:10.1016/j.anzjph.2022.100013. PMID 36641959.
- ↑ "Electronic Cigarettes – Are e-cigarettes less harmful than regular cigarettes?". Centers for Disease Control and Prevention. 2 November 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Smith, L; Brar, K; Srinivasan, K; Enja, M; Lippmann, S (June 2016). "E-cigarettes: How "safe" are they?". J Fam Pract. 65 (6): 380–5. PMID 27474819.
- ↑ 114.0 114.1 114.2 114.3 114.4 Mescolo, Federica; Ferrante, Giuliana; La Grutta, Stefania (20 August 2021). "Effects of E-Cigarette Exposure on Prenatal Life and Childhood Respiratory Health: A Review of Current Evidence". Frontiers in Pediatrics. 9. doi:10.3389/fped.2021.711573. PMC 8430837. PMID 34513764.
 This article incorporates text by Federica Mescolo, Giuliana Ferrante, and Stefania La Grutta available under the CC BY 4.0 license.
This article incorporates text by Federica Mescolo, Giuliana Ferrante, and Stefania La Grutta available under the CC BY 4.0 license.
- ↑ 115.0 115.1 Grana, Rachel; Benowitz, Neal; Glantz, Stanton A. (13 May 2014). "E-Cigarettes: A Scientific Review". Circulation. 129 (19): 1972–1986. doi:10.1161/circulationaha.114.007667. PMC 4018182. PMID 24821826.
- ↑ 116.00 116.01 116.02 116.03 116.04 116.05 116.06 116.07 116.08 116.09 116.10 116.11 116.12 116.13 116.14 116.15 116.16 116.17 116.18 116.19 116.20 116.21 116.22 116.23 116.24 116.25 116.26 116.27 116.28 116.29 116.30 116.31 116.32 116.33 116.34 116.35 116.36 116.37 116.38 116.39 116.40 116.41 116.42 116.43 Xue, Jiaping; Yang, Suping; Seng, Seyha (14 May 2014). "Mechanisms of Cancer Induction by Tobacco-Specific NNK and NNN". Cancers. 6 (2): 1138–1156. doi:10.3390/cancers6021138. PMC 4074821. PMID 24830349.
 This article incorporates text by Jiaping Xue, Suping Yang, and Seyha Seng available under the CC BY 3.0 license.
This article incorporates text by Jiaping Xue, Suping Yang, and Seyha Seng available under the CC BY 3.0 license.
- ↑ Xu, Dongmei; Lusso, Marcos F.; Strickland, James A. (2020). "Impact of Genetics and Production Practices on Tobacco-Specific Nitrosamine Formation". The Tobacco Plant Genome. Springer International Publishing. pp. 157–174. ISBN 978-3-030-29493-9.
- ↑ 118.0 118.1 Zeng, Ting; Liu, Yanxia; Jiang, Yingfang; Zhang, Lan; Zhang, Yagang; Zhao, Lin; Jiang, Xiaoli; Zhang, Qiang (August 2023). "Advanced Materials Design for Adsorption of Toxic Substances in Cigarette Smoke". Advanced Science. 10 (22). doi:10.1002/advs.202301834. PMC 10401148. PMID 37211707.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Ting Zeng, Yanxia Liu, Yingfang Jiang, Lan Zhang, Yagang Zhang, Lin Zhao, Xiaoli Jiang, and Qiang Zhang available under the CC BY 4.0 license.
This article incorporates text by Ting Zeng, Yanxia Liu, Yingfang Jiang, Lan Zhang, Yagang Zhang, Lin Zhao, Xiaoli Jiang, and Qiang Zhang available under the CC BY 4.0 license.
- ↑ Zenkner, Fernanda Fleig; Margis-Pinheiro, Márcia; Cagliari, Alexandro (1 April 2019). "Nicotine Biosynthesis in Nicotiana : A Metabolic Overview". Tobacco Science. 56 (1): 1–9. doi:10.3381/18-063.
- ↑ 120.0 120.1 Shoji, Tsubasa; Hashimoto, Takashi; Saito, Kazuki (14 March 2024). "Genetic regulation and manipulation of nicotine biosynthesis in tobacco: strategies to eliminate addictive alkaloids". Journal of Experimental Botany. 75 (6): 1741–1753. doi:10.1093/jxb/erad341. PMC 10938045. PMID 37647764.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Tsubasa Shoji, Takashi Hashimoto, and Kazuki Saito available under the CC BY 4.0 license.
This article incorporates text by Tsubasa Shoji, Takashi Hashimoto, and Kazuki Saito available under the CC BY 4.0 license.
- ↑ Fitzpatrick, Paul F (31 August 2018). "The enzymes of microbial nicotine metabolism". Beilstein Journal of Organic Chemistry. 14: 2295–2307. doi:10.3762/bjoc.14.204. PMC 6122326. PMID 30202483.
 This article incorporates text by Paul F Fitzpatrick available under the CC BY 4.0 license.
This article incorporates text by Paul F Fitzpatrick available under the CC BY 4.0 license.
- ↑ 122.0 122.1 122.2 122.3 Hecht, Stephen S. (March 1999). "DNA adduct formation from tobacco-specific N-nitrosamines". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 424 (1–2): 127–142. doi:10.1016/s0027-5107(99)00014-7. PMID 10064856.
- ↑ 123.0 123.1 123.2 Li, Yupeng; Hecht, Stephen S. (4 May 2022). "Metabolism and DNA Adduct Formation of Tobacco-Specific N-Nitrosamines". International Journal of Molecular Sciences. 23 (9): 5109. doi:10.3390/ijms23095109. PMC 9104174. PMID 35563500.
 This article incorporates text by Yupeng Li and Stephen S. Hecht available under the CC BY 4.0 license.
This article incorporates text by Yupeng Li and Stephen S. Hecht available under the CC BY 4.0 license.
- ↑ 124.000 124.001 124.002 124.003 124.004 124.005 124.006 124.007 124.008 124.009 124.010 124.011 124.012 124.013 124.014 124.015 124.016 124.017 124.018 124.019 124.020 124.021 124.022 124.023 124.024 124.025 124.026 124.027 124.028 124.029 124.030 124.031 124.032 124.033 124.034 124.035 124.036 124.037 124.038 124.039 124.040 124.041 124.042 124.043 124.044 124.045 124.046 124.047 124.048 124.049 124.050 124.051 124.052 124.053 124.054 124.055 124.056 124.057 124.058 124.059 124.060 124.061 124.062 124.063 124.064 124.065 124.066 124.067 124.068 124.069 124.070 124.071 124.072 124.073 124.074 124.075 124.076 124.077 124.078 124.079 124.080 124.081 124.082 124.083 124.084 124.085 124.086 124.087 124.088 124.089 124.090 124.091 124.092 124.093 124.094 124.095 124.096 124.097 124.098 124.099 124.100 124.101 124.102 124.103 124.104 124.105 124.106 124.107 124.108 124.109 124.110 124.111 124.112 124.113 124.114 124.115 124.116 124.117 124.118 124.119 124.120 124.121 124.122 124.123 124.124 124.125 124.126 124.127 124.128 124.129 124.130 Cheng, Wan-Li; Chen, Kuan-Yuan; Lee, Kang-Yun; Feng, Po-Hao; Wu, Sheng-Ming (2020). "Nicotinic-nAChR signaling mediates drug resistance in lung cancer". Journal of Cancer. 11 (5): 1125–1140. doi:10.7150/jca.36359. PMC 6959074. PMID 31956359.
 This article incorporates text by Wan-Li Cheng, Kuan-Yuan Chen, Kang-Yun Lee, Po-Hao Feng, and Sheng-Ming Wunderground available under the CC BY 4.0 license.
This article incorporates text by Wan-Li Cheng, Kuan-Yuan Chen, Kang-Yun Lee, Po-Hao Feng, and Sheng-Ming Wunderground available under the CC BY 4.0 license.
- ↑ Gupta, Alpana K.; Tulsyan, Sonam; Bharadwaj, Mausumi; Mehrotra, Ravi (February 2019). "Grass roots approach to control levels of carcinogenic nitrosamines, NNN and NNK in smokeless tobacco products". Food and Chemical Toxicology. 124: 359–366. doi:10.1016/j.fct.2018.12.011. PMID 30543893.
- ↑ "Cannabis (Marijuana) Smoke". California Office of Environmental Health Hazard Assessment. 2025.
- ↑ "THC (Δ⁹-Tetrahydrocannabinol or Delta-9-Tetrahydrocannabinol)". California Office of Environmental Health Hazard Assessment. 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Electronic Cigarettes (E-Cigarettes)". California Office of Environmental Health Hazard Assessment. 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "For Businesses". California Office of Environmental Health Hazard Assessment. 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Feng, Lida; Huang, Guixiao; Peng, Lei; Liang, Rui; Deng, Dashi; Zhang, Shaohua; Li, Guangzhi; Wu, Song (23 April 2024). "Comparison of bladder carcinogenesis biomarkers in the urine of traditional cigarette users and e-cigarette users". Frontiers in Public Health. 12. doi:10.3389/fpubh.2024.1385628. PMC 11075070. PMID 38716244.
{{cite journal}}: Check|pmc=value (help) - ↑ Seiler-Ramadas, Radhika; Sandner, Isabell; Haider, Sandra; Grabovac, Igor; Dorner, Thomas Ernst (2020). "Health effects of electronic cigarette (e‑cigarette) use on organ systems and its implications for public health". Wiener klinische Wochenschrift. doi:10.1007/s00508-020-01711-z. ISSN 0043-5325. PMID 32691214.
 This article incorporates text by Radhika Seiler-Ramadas, Isabell Sandner, Sandra Haider, Igor Grabovac, and Thomas Ernst Dorner available under the CC BY 4.0 license.
This article incorporates text by Radhika Seiler-Ramadas, Isabell Sandner, Sandra Haider, Igor Grabovac, and Thomas Ernst Dorner available under the CC BY 4.0 license.
- ↑ Brooks, Arrin C.; Henderson, Brandon J. (29 January 2021). "Systematic Review of Nicotine Exposure's Effects on Neural Stem and Progenitor Cells". Brain Sciences. 11 (2): 172. doi:10.3390/brainsci11020172. PMID 33573081.
 This article incorporates text by Arrin C. Brooks and Brandon J. Henderson available under the CC BY 4.0 license.
This article incorporates text by Arrin C. Brooks and Brandon J. Henderson available under the CC BY 4.0 license.
- ↑ Wu, Liang; Wang, Li'ao; Yang, Jun; Jia, Wenqing; Xu, Yulun (31 March 2022). "Clinical Features, Treatments, and Prognosis of Intramedullary Spinal Cord Metastases From Lung Cancer: A Case Series and Systematic Review". Neurospine. 19 (1): 65–76. doi:10.14245/ns.2142910.455. PMC 8987539. PMID 35130420.
- ↑ Di Vita, Maria (2013). "Strong correlation between diet and development of colorectal cancer". Frontiers in Bioscience. 18 (1): 190. doi:10.2741/4095. PMID 23276917.
- ↑ Russo, Patrizia; Cardinale, Alessio; Margaritora, Stefano; Cesario, Alfredo (November 2012). "Nicotinic receptor and tobacco-related cancer". Life Sciences. 91 (21–22): 1087–1092. doi:10.1016/j.lfs.2012.05.003. PMID 22705232.
- ↑ Chu, Kent-Man; H. Cho, Chi; Y. Shin, Vivian (1 November 2012). "Nicotine and Gastrointestinal Disorders: Its Role in Ulceration and Cancer Development". Current Pharmaceutical Design. 19 (1): 5–10. doi:10.2174/13816128130103. PMID 22950507.
- ↑ "What You Can Do to Protect Youth From the Harms of Vaping". Centers for Disease Control and Prevention. 18 September 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Vaping Devices (Electronic Cigarettes) Drug". National Institute on Drug Abuse. National Institutes of Health. January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 139.0 139.1 Maan, Meenu; Abuzayeda, Moosa; Kaklamanos, Eleftherios G.; Jamal, Mohamed; Dutta, Mainak; Moharamzadeh, Keyvan (13 April 2023). "Molecular insights into the role of electronic cigarettes in oral carcinogenesis". Critical Reviews in Toxicology. 0 (0): 1–14. doi:10.1080/10408444.2023.2190764. PMID 37051806.
- ↑ Sultan, Ahmed S.; Jessri, Maryam; Farah, Camile S. (March 2021). "Electronic nicotine delivery systems: Oral health implications and oral cancer risk". Journal of Oral Pathology & Medicine. doi:10.1111/jop.12810. ISSN 0904-2512. PMID 30507043.
- ↑ Yeh, Kristen; Li, Li; Wania, Frank; Abbatt, Jonathan P.D. (February 2022). "Thirdhand smoke from tobacco, e-cigarettes, cannabis, methamphetamine and cocaine: Partitioning, reactive fate, and human exposure in indoor environments". Environment International. 160: 107063. doi:10.1016/j.envint.2021.107063. PMID 34954646.
- ↑ Almeida-da-Silva, Cássio Luiz Coutinho; Matshik Dakafay, Harmony; O'Brien, Kenji; Montierth, Dallin; Xiao, Nan; Ojcius, David M. (2020). "Effects of electronic cigarette aerosol exposure on oral and systemic health". Biomedical Journal. doi:10.1016/j.bj.2020.07.003. ISSN 2319-4170. PMC 8358192. PMID 33039378.
- ↑ Addo Ntim, Susana; Martin, Bria; Termeh-Zonoozi, Yasmin (20 October 2022). "Review of Use Prevalence, Susceptibility, Advertisement Exposure, and Access to Electronic Nicotine Delivery Systems among Minorities and Low-Income Populations in the United States". International Journal of Environmental Research and Public Health. 19 (20): 13585. doi:10.3390/ijerph192013585. PMC 9603140. PMID 36294164.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Susana Addo Ntim, Bria Martin, and Yasmin Termeh-Zonoozi available under the CC BY 4.0 license.
This article incorporates text by Susana Addo Ntim, Bria Martin, and Yasmin Termeh-Zonoozi available under the CC BY 4.0 license.
- ↑ Tobacco smoke and involuntary smoking. 2004. pp. 1–1438. ISBN 978-92-832-1283-6.
- ↑ 145.0 145.1 145.2 145.3 145.4 145.5 145.6 145.7 "General Information About Secondhand Smoke". Centers for Disease Control and Prevention. 14 September 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Fairchild, Amy Lauren; Bayer, Ronald; Lee, Ju Sung (July 2019). "The E-Cigarette Debate: What Counts as Evidence?". American Journal of Public Health. 109 (7): 1000–1006. doi:10.2105/AJPH.2019.305107. PMC 6603453. PMID 31095415.
- ↑ Perez-Warnisher, M. Teresa; De Miguel, M. del Pilar Carballosa; Seijo, Luis M. (5 November 2018). "Tobacco Use Worldwide: Legislative Efforts to Curb Consumption". Annals of Global Health. 84 (4): 571. doi:10.29024/aogh.2362. PMC 6748295. PMID 30779502.
 This article incorporates text by M. Teresa Perez-Warnisher, M. Pilar Carballosa de Miguel, and Luis M. Seijo available under the CC BY 4.0 license.
This article incorporates text by M. Teresa Perez-Warnisher, M. Pilar Carballosa de Miguel, and Luis M. Seijo available under the CC BY 4.0 license.
- ↑ 148.0 148.1 Kaunelienė, Violeta; Meišutovič-Akhtarieva, Marija; Martuzevičius, Dainius (September 2018). "A review of the impacts of tobacco heating system on indoor air quality versus conventional pollution sources". Chemosphere. 206: 568–578. Bibcode:2018Chmsp.206..568K. doi:10.1016/j.chemosphere.2018.05.039. ISSN 0045-6535. PMID 29778082.
- ↑ "Quitting E-cigarettes". American Cancer Society. 10 October 2020.
- ↑ "Adult Smoking Cessation—The Use of E-Cigarettes". Centers for Disease Control and Prevention. 25 October 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 151.0 151.1 "Quit Don't Switch". American Lung Association. 28 September 2023.
- ↑ Lyzwinski, Lynnette Nathalie; Naslund, John A.; Miller, Christopher J.; Eisenberg, Mark J. (11 April 2022). "Global youth vaping and respiratory health: epidemiology, interventions, and policies". npj Primary Care Respiratory Medicine. 32 (1): 14. doi:10.1038/s41533-022-00277-9. PMC 9001701. PMID 35410990.
 This article incorporates text by Lynnette Nathalie Lyzwinski, John A. Naslund, Christopher J. Miller, and Mark J. Eisenberg available under the CC BY 4.0 license.
This article incorporates text by Lynnette Nathalie Lyzwinski, John A. Naslund, Christopher J. Miller, and Mark J. Eisenberg available under the CC BY 4.0 license.
- ↑ Jenssen, Brian P.; Walley, Susan C.; Groner, Judith A.; Rahmandar, Maria; Boykan, Rachel; Mih, Bryan; Marbin, Jyothi N.; Caldwell, Alice Little (1 February 2019). "E-Cigarettes and Similar Devices". Pediatrics. 143 (2). doi:10.1542/peds.2018-3652. PMC 6644065. PMID 30835247.
- ↑ Widysanto A, Combest FE, Dhakal A, Saadabadi A (10 November 2020). "Nicotine Addiction". StatPearls Publishing. PMID 29763090.
- ↑ 155.0 155.1 Nansseu, Jobert Richie N.; Bigna, Jean Joel R. (25 December 2016). "Electronic Cigarettes for Curbing the Tobacco-Induced Burden of Noncommunicable Diseases: Evidence Revisited with Emphasis on Challenges in Sub-Saharan Africa". Pulmonary Medicine. 2016: 1–9. doi:10.1155/2016/4894352. ISSN 2090-1836. PMC 5220510. PMID 28116156.
 This article incorporates text by Jobert Richie N. Nansseu and Jean Joel R. Bigna available under the CC BY 4.0 license.
This article incorporates text by Jobert Richie N. Nansseu and Jean Joel R. Bigna available under the CC BY 4.0 license.
- ↑ 156.0 156.1 156.2 156.3 Brandt, Allan M. (January 2012). "Inventing Conflicts of Interest: A History of Tobacco Industry Tactics". American Journal of Public Health. 102 (1): 63–71. doi:10.2105/AJPH.2011.300292. PMC 3490543. PMID 22095331.
- ↑ 157.0 157.1 Stone, Emily; Marshall, Henry (2019). "Tobacco and electronic nicotine delivery systems regulation". Translational Lung Cancer Research. 8 (S1): S67–S76. doi:10.21037/tlcr.2019.03.13. ISSN 2218-6751. PMC 6546633. PMID 31211107.
- ↑ 158.0 158.1 158.2 158.3 Yan, Duo; Wang, Zicheng; Laestadius, Linnea; Mosalpuria, Kavita; Wilson, Fernando A; Yan, Alice; Lv, Xiaoyang; Zhang, Xiaotian; Bhuyan, Soumitra S; Wang, Yang (25 August 2023). "A systematic review for the impacts of global approaches to regulating electronic nicotine products". Journal of Global Health. 13. doi:10.7189/jogh.13.04076. PMC 10451104. PMID 37622721.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Duo Yan, Zicheng Wang, Linnea Laestadius, Kavita Mosalpuria, Fernando A Wilson, Alice Yan, Xiaoyang Lv, Xiaotian Zhang, Soumitra S Bhuyan, and Yang Wang available under the CC BY 3.0 license.
This article incorporates text by Duo Yan, Zicheng Wang, Linnea Laestadius, Kavita Mosalpuria, Fernando A Wilson, Alice Yan, Xiaoyang Lv, Xiaotian Zhang, Soumitra S Bhuyan, and Yang Wang available under the CC BY 3.0 license.
- ↑ 159.00 159.01 159.02 159.03 159.04 159.05 159.06 159.07 159.08 159.09 159.10 Emma, Rosalia; Caruso, Massimo; Campagna, Davide; Pulvirenti, Roberta; Li Volti, Giovanni (September 2022). "The Impact of Tobacco Cigarettes, Vaping Products and Tobacco Heating Products on Oxidative Stress". Antioxidants. 11 (9): 1829. doi:10.3390/antiox11091829. PMC 9495690. PMID 36139904.
 This article incorporates text by Rosalia Emma, Massimo Caruso, Davide Campagna, Roberta Pulvirenti, and Giovanni Li Volti available under the CC BY 4.0 license.
This article incorporates text by Rosalia Emma, Massimo Caruso, Davide Campagna, Roberta Pulvirenti, and Giovanni Li Volti available under the CC BY 4.0 license.
- ↑ 160.00 160.01 160.02 160.03 160.04 160.05 160.06 160.07 160.08 160.09 160.10 160.11 160.12 160.13 160.14 160.15 160.16 160.17 160.18 160.19 160.20 160.21 160.22 160.23 160.24 160.25 160.26 160.27 160.28 160.29 160.30 160.31 160.32 160.33 160.34 160.35 160.36 160.37 160.38 160.39 160.40 160.41 160.42 160.43 160.44 160.45 160.46 160.47 160.48 Auschwitz, Emily; Almeda, Jasmine; Andl, Claudia D. (31 October 2023). "Mechanisms of E-Cigarette Vape-Induced Epithelial Cell Damage". Cells. 12 (21): 2552. doi:10.3390/cells12212552. PMC 10650279. PMID 37947630.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Emily Auschwitz, Jasmine Almeda, and Claudia D. Andl available under the CC BY 4.0 license.
This article incorporates text by Emily Auschwitz, Jasmine Almeda, and Claudia D. Andl available under the CC BY 4.0 license.
- ↑ Chadi, Nicholas; Vyver, Ellie; Bélanger, Richard E (17 September 2021). "Protecting children and adolescents against the risks of vaping". Paediatrics & Child Health. 26 (6): 358–365. doi:10.1093/pch/pxab037. PMC 8448493. PMID 34552676.
- ↑ Braillon, Alain; Lang, Adam Edward (April 2023). "The International Agency for Research on Cancer and e-cigarette carcinogenicity: time for an evaluation". European Journal of Epidemiology. 38 (4): 391–391. doi:10.1007/s10654-023-00993-7. PMID 36961667.
- ↑ Breland, Alison; Soule, Eric; Lopez, Alexa; Ramôa, Carolina; El-Hellani, Ahmad; Eissenberg, Thomas (2017). "Electronic cigarettes: what are they and what do they do?". Annals of the New York Academy of Sciences. 1394 (1): 5–30. Bibcode:2017NYASA1394....5B. doi:10.1111/nyas.12977. ISSN 0077-8923. PMC 4947026. PMID 26774031.
- ↑ 164.0 164.1 164.2 Improgo, M R D; Scofield, M D; Tapper, A R; Gardner, P D (2 September 2010). "From smoking to lung cancer: the CHRNA5/A3/B4 connection". Oncogene. 29 (35): 4874–4884. doi:10.1038/onc.2010.256. PMC 3934347. PMID 20581870.
- ↑ Zhao, Yue (2016). "The Oncogenic Functions of Nicotinic Acetylcholine Receptors". Journal of Oncology. 2016: 1–9. doi:10.1155/2016/9650481. PMC 4769750. PMID 26981122.
 This article incorporates text by Yue Zhao available under the CC BY 4.0 license.
This article incorporates text by Yue Zhao available under the CC BY 4.0 license.
- ↑ Sarlak, Saharnaz; Lalou, Claude; Amoedo, Nivea Dias; Rossignol, Rodrigue (February 2020). "Metabolic reprogramming by tobacco-specific nitrosamines (TSNAs) in cancer". Seminars in Cell & Developmental Biology. 98: 154–166. doi:10.1016/j.semcdb.2019.09.001. PMID 31699542.
- ↑ Al-Fayez, Sarah; El-Metwally, Ashraf (6 February 2023). "Cigarette smoking and prostate cancer: A systematic review and meta-analysis of prospective cohort studies". Tobacco Induced Diseases. 21 (February): 1–12. doi:10.18332/tid/157231. PMC 9900478. PMID 36762260.
{{cite journal}}: Check|pmc=value (help) - ↑ Owen, Kelly P.; Sutter, Mark E.; Albertson, Timothy E. (February 2014). "Marijuana: Respiratory Tract Effects". Clinical Reviews in Allergy & Immunology. 46 (1): 65–81. doi:10.1007/s12016-013-8374-y. PMID 23715638.
- ↑ 169.0 169.1 169.2 Gakidou, Emmanuela (June 2021). "Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019". The Lancet. 397 (10292): 2337–2360. doi:10.1016/S0140-6736(21)01169-7. PMC 8223261. PMID 34051883.
 This article incorporates text by Emmanuela Gakidou available under the CC BY 4.0 license.
This article incorporates text by Emmanuela Gakidou available under the CC BY 4.0 license.
- ↑ Lee, Elizabeth; Kazerooni, Ella A. (December 2022). "Lung Cancer Screening". Seminars in Respiratory and Critical Care Medicine. 43 (06): 839–850. doi:10.1055/s-0042-1757885. PMID 36442474.
- ↑ Bade, Brett C.; Dela Cruz, Charles S. (March 2020). "Lung Cancer 2020". Clinics in Chest Medicine. 41 (1): 1–24. doi:10.1016/j.ccm.2019.10.001. PMID 23566962.
- ↑ 172.0 172.1 Leiter, Amanda; Veluswamy, Rajwanth R.; Wisnivesky, Juan P. (September 2023). "The global burden of lung cancer: current status and future trends". Nature Reviews Clinical Oncology. 20 (9): 624–639. doi:10.1038/s41571-023-00798-3. PMID 37479810.
- ↑ Zarnke, Andrew M.; Tharmalingam, Sujeenthar; Boreham, Douglas R.; Brooks, Antone L. (March 2019). "BEIR VI radon: The rest of the story". Chemico-Biological Interactions. 301: 81–87. doi:10.1016/j.cbi.2018.11.012. PMID 30763549.
- ↑ 174.0 174.1 Madas, Balázs G.; Boei, Jan; Fenske, Nora; Hofmann, Werner; Mezquita, Laura (November 2022). "Effects of spatial variation in dose delivery: what can we learn from radon-related lung cancer studies?". Radiation and Environmental Biophysics. 61 (4): 561–577. doi:10.1007/s00411-022-00998-y. PMC 9630403. PMID 36208308.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Balázs G. Madas, Jan Boei, Nora Fenske, Werner Hofmann, and Laura Mezquita available under the CC BY 4.0 license.
This article incorporates text by Balázs G. Madas, Jan Boei, Nora Fenske, Werner Hofmann, and Laura Mezquita available under the CC BY 4.0 license.
- ↑ Eidy, Mountaha; Regina, Angela C.; Tishkowski, Kevin (January 2024). "Radon Toxicity". StatPearls. StatPearls Publishing.
- ↑ Fuente, Marta; Long, Stephanie; Fenton, David; Hung, Le Chi; Goggins, Jamie; Foley, Mark (September 2020). "Review of recent radon research in Ireland, OPTI-SDS project and its impact on the National Radon Control Strategy". Applied Radiation and Isotopes. 163: 109210. doi:10.1016/j.apradiso.2020.109210. PMID 32561049.
- ↑ Giraldo-Osorio, Alexandra; Ruano-Ravina, Alberto; Varela-Lema, Leonor; Barros-Dios, Juan M.; Pérez-Ríos, Mónica (24 June 2020). "Residential Radon in Central and South America: A Systematic Review". International Journal of Environmental Research and Public Health. 17 (12): 4550. doi:10.3390/ijerph17124550. PMC 7345538. PMID 32599800.
- ↑ Folesani, Giuseppina; Galetti, Maricla; Petronini, Pier Giorgio; Mozzoni, Paola; La Monica, Silvia; Cavallo, Delia; Corradi, Massimo (25 February 2023). "Interaction between Occupational and Non-Occupational Arsenic Exposure and Tobacco Smoke on Lung Cancerogenesis: A Systematic Review". International Journal of Environmental Research and Public Health. 20 (5): 4167. doi:10.3390/ijerph20054167. PMC 10001869. PMID 36901176.
{{cite journal}}: Check|pmc=value (help) - ↑ 179.0 179.1 179.2 179.3 179.4 179.5 179.6 AbdelRahman, Rania T.; Kamal, Nurkhalida; Mediani, Ahmed; Farag, Mohamed A. (20 December 2022). "How Do Herbal Cigarettes Compare To Tobacco? A Comprehensive Review of Their Sensory Characters, Phytochemicals, and Functional Properties". ACS Omega. 7 (50): 45797–45809. doi:10.1021/acsomega.2c04708. PMC 9773184. PMID 36570239.
{{cite journal}}: Check|pmc=value (help) - ↑ 180.0 180.1 Mavroeidis, Antonios; Stavropoulos, Panteleimon; Papadopoulos, George; Tsela, Aikaterini; Roussis, Ioannis; Kakabouki, Ioanna (15 January 2024). "Alternative Crops for the European Tobacco Industry: A Systematic Review". Plants. 13 (2): 236. doi:10.3390/plants13020236. PMC 10818552. PMID 38256796.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Antonios Mavroeidis, Panteleimon Stavropoulos, George Papadopoulos, Aikaterini Tsela, Ioannis Roussis, and Ioanna Kakabouki available under the CC BY 4.0 license.
This article incorporates text by Antonios Mavroeidis, Panteleimon Stavropoulos, George Papadopoulos, Aikaterini Tsela, Ioannis Roussis, and Ioanna Kakabouki available under the CC BY 4.0 license.
- ↑ Kaur, Gurjot; Muthumalage, Thivanka; Rahman, Irfan (2018). "Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products". Toxicology Letters. 288: 143–155. doi:10.1016/j.toxlet.2018.02.025. ISSN 0378-4274. PMC 6549714. PMID 29481849.
- ↑ 182.0 182.1 "Cancer". Centers for Disease Control and Prevention. 19 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 183.0 183.1 183.2 Underner, M.; Urban, T.; Perriot, J.; de Chazeron, I.; Meurice, J.-C. (June 2014). "Cannabis et cancer bronchique" [Cannabis smoking and lung cancer]. Revue des Maladies Respiratoires (in French). 31 (6): 488–498. doi:10.1016/j.rmr.2013.12.002. PMID 25012035.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ 184.0 184.1 184.2 184.3 184.4 Greydanus, Donald E.; Hawver, Elizabeth K.; Greydanus, Megan M.; Merrick, Joav (2013). "Marijuana: Current Concepts†". Frontiers in Public Health. 1. doi:10.3389/fpubh.2013.00042. PMC 3859982. PMID 24350211.
 This article incorporates text by Donald E Greydanus, Elizabeth K Hawver, Megan M Greydanus, and Joav Merrick available under the CC BY 4.0 license.
This article incorporates text by Donald E Greydanus, Elizabeth K Hawver, Megan M Greydanus, and Joav Merrick available under the CC BY 4.0 license.
- ↑ 185.0 185.1 185.2 Huang, Peng; Zhang, Peng Fei; Li, Qiu (September 2023). "Causal relationship between cannabis use and cancer: a genetically informed perspective". Journal of Cancer Research and Clinical Oncology. 149 (11): 8631–8638. doi:10.1007/s00432-023-04807-x. PMID 37099198.
- ↑ Joshi, Manish; Joshi, Anita; Bartter, Thaddeus (March 2014). "Marijuana and lung diseases:". Current Opinion in Pulmonary Medicine. 20 (2): 173–179. doi:10.1097/MCP.0000000000000026. PMID 24384575.
- ↑ 187.0 187.1 Lipari, R. N; Van Horn, S. L (31 May 2017). "Trends in Smokeless Tobacco Use and Initiation: 2002 to 2014". Substance Abuse and Mental Health Services Administration. PMID 28636307.
{{cite journal}}: Cite journal requires|journal=(help) This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 188.0 188.1 188.2 188.3 188.4 Drope, Jeffrey; Cahn, Zachary; Kennedy, Rosemary; Liber, Alex C.; Stoklosa, Michal; Henson, Rosemarie; Douglas, Clifford E.; Drope, Jacqui (2017). "Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine". CA: A Cancer Journal for Clinicians. 67 (6): 449–471. doi:10.3322/caac.21413. ISSN 0007-9235. PMID 28961314.
- ↑ 189.0 189.1 189.2 189.3 Ye, Dongxia; Rahman, Irfan (10 February 2023). "Emerging Oral Nicotine Products and Periodontal Diseases". International Journal of Dentistry. 2023: 1–7. doi:10.1155/2023/9437475. PMC 9937772. PMID 36819641.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Dongxia Ye and Irfan Rahman available under the CC BY 4.0 license.
This article incorporates text by Dongxia Ye and Irfan Rahman available under the CC BY 4.0 license.
- ↑ 190.0 190.1 Valen, Håkon; Becher, Rune; Vist, Gunn Elisabeth; Holme, Jørn Andreas; Mdala, Ibrahimu; Elvsaas, Ida‐Kristin Ørjasæter; Alexander, Jan; Underland, Vigdis; Brinchmann, Bendik Christian; Grimsrud, Tom Kristian (15 December 2023). "A systematic review of cancer risk among users of smokeless tobacco (Swedish snus) exclusively, compared with no use of tobacco". International Journal of Cancer. 153 (12): 1942–1953. doi:10.1002/ijc.34643. PMID 37480210.
Further reading
- "Cancer". World Health Organization. 3 February 2022.
- Sansone, Luigi; Milani, Francesca; Fabrizi, Riccardo; Belli, Manuel; Cristina, Mario; Zagà, Vincenzo; de Iure, Antonio; Cicconi, Luca; Bonassi, Stefano; Russo, Patrizia (26 September 2023). "Nicotine: From Discovery to Biological Effects". International Journal of Molecular Sciences. 24 (19): 14570. doi:10.3390/ijms241914570. PMC 10572882. PMID 37834017.
{{cite journal}}: Check|pmc=value (help) - Kotewar, Samrudhi S; Pakhale, Aayushi; Tiwari, Rupali; Reche, Amit; Singi, Shriya R (13 August 2023). "Electronic Nicotine Delivery System: End to Smoking or Just a New Fancy Cigarette". Cureus. doi:10.7759/cureus.43425. PMC 10497069. PMID 37706142.
{{cite journal}}: Check|pmc=value (help) - Stewart, Bernard W. (2019). "Mechanisms of carcinogenesis: from initiation and promotion to the hallmarks". Tumour Site Concordance and Mechanisms of Carcinogenesis. International Agency for Research on Cancer. ISBN 978-92-832-2217-0.
- Birkett, Nicholas; Al-Zoughool, Mustafa; Bird, Michael; Baan, Robert A.; Zielinski, Jan; Krewski, Daniel (17 November 2019). "Overview of biological mechanisms of human carcinogens". Journal of Toxicology and Environmental Health, Part B. 22 (7–8): 288–359. doi:10.1080/10937404.2019.1643539. PMID 31631808.
- Cogliano, V. J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; Wild, C. P. (21 December 2011). "Preventable Exposures Associated With Human Cancers". Journal of the National Cancer Institute. 103 (24): 1827–1839. doi:10.1093/jnci/djr483. PMC 3243677. PMID 22158127.