Peritoneal dialysis
| Peritoneal dialysis | |
|---|---|
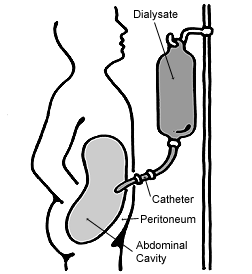 | |
| Diagram of peritoneal dialysis | |
| Specialty | Nephrology |
| Indications | Kidney failure[1] |
| Contraindications | Extensive prior abdominal surgery, inflammatory bowel disease[1] |
| Complications | Infections within the abdomen, hernias, high blood sugar, bleeding in the abdomen, blockage of the catheter[1] |
Peritoneal dialysis (PD) is a type of dialysis which uses the peritoneum in a person's abdomen as the membrane through which fluid and dissolved substances are exchanged with the blood.[2] It is used to remove excess fluid, correct electrolyte problems, and remove toxins in those with kidney failure.[1] Peritoneal dialysis has better outcomes than hemodialysis during the first couple of years.[3] Other benefits include greater flexibility and better tolerability in those with significant heart disease.[3]
Complications may include infections within the abdomen, hernias, high blood sugar, bleeding in the abdomen, and blockage of the catheter.[1] Use is not possible in those with extensive prior abdominal surgery or inflammatory bowel disease.[1] It requires some degree of technical skill to be done properly.[3]
In peritoneal dialysis, a specific solution is introduced through a permanent tube in the lower abdomen and then removed.[1] This may either occur at regular intervals throughout the day, known as continuous ambulatory dialysis, or at night with the assistance of a machine, known as automated peritoneal dialysis.[1] The solution is typically made of sodium chloride, hydrogen carbonate, and an osmotic agent such as glucose.[1]
Peritoneal dialysis was first carried out in the 1920s; however, long term use did not come into medical practice until the 1960s.[4] The solution used for peritoneal dialysis is on the World Health Organization's List of Essential Medicines.[5] The cost of dialysis solution in the developing world is about US$6.77–7.30 per two-liter bag or about $12,000 per year.[6][7] In the United States peritoneal dialysis costs the government about $53,400 per person per year.[3] As of 2009 peritoneal dialysis was available in 12 out of 53 African countries.[7]
Health effects
PD is less efficient at removing wastes from the body than hemodialysis, and the presence of the tube presents a risk of peritonitis due to the potential to introduce bacteria to the abdomen.[8] There is not sufficient evidence to be clear about the best treatment for PD-associated peritonitis, although direct infusion of antibiotics into the peritoneum appears to offer slight advantage over the intravenous route of administration; there is no clear advantage for other frequently used treatments such as routine peritoneal lavage or use of urokinase.[9] The use of preventative nasal mupirocin is of unclear effect with respect to peritonitis.[10] Infections can be as frequent as once every 15 months (0.8 episodes per patient year). Compared to hemodialysis, PD allows greater patient mobility, produces fewer swings in symptoms due to its continuous nature, and phosphate compounds are better removed, but large amounts of albumin are removed which requires constant monitoring of nutritional status. The costs of PD are generally lower than those of HD in most parts of the world, this cost advantage is most apparent in developed economies.[11] There is insufficient research to adequately compare the risks and benefits between CAPD and APD; a Cochrane Review of three small clinical trials found no difference in clinically important outcomes (i.e. morbidity or mortality) for patients with end stage renal disease, nor was there any advantage in preserving the functionality of the kidneys. The results suggested APD may have psychosocial advantages for younger patients and those who are employed or pursuing an education.[12]
Other complications include hypotension (due to excess fluid exchange and sodium removal), low back pain and hernia or leaking fluid due to high pressure within the abdomen. PD may also be used for patients with cardiac instability as it does not result in rapid and significant alterations to body fluids, and for patients with insulin-dependent diabetes mellitus due to the inability to control blood sugar levels through the catheter. Hypertriglyceridemia and obesity are also concerns due to the large volume of glucose in the fluid, which can add 500-1200 calories to the diet per day.[13] Of the three types of connection and fluid exchange systems (standard, twin-bag and y-set; the latter two involving two bags and only one connection to the catheter, the y-set uses a single y-shaped connection between the bags involving emptying, flushing out then filling the peritoneum through the same connection) the twin-bag and y-set systems were found superior to conventional systems at preventing peritonitis.[14]
Method
Best practices for peritoneal dialysis state that before peritoneal dialysis should be implemented, the person's understanding of the process and support systems should be assessed, with education on how to care for the catheter and to address any gaps in understanding that may exist. The person should receive ongoing monitoring to ensure adequate dialysis, and be regularly assessed for complications. Finally, they should be educated on the importance of infection control and an appropriate medical regimen established with their cooperation.[15]
- Dialysis process
-
Hookup
-
Infusion
-
Diffusion (fresh)
-
Diffusion (waste)
-
Drainage
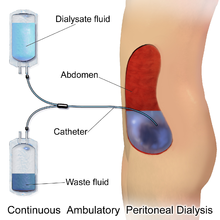
The abdomen is cleaned in preparation for surgery and a catheter is surgically inserted with one end in the abdomen and the other protruding from the skin.[16] Before each infusion the catheter must be cleaned, and flow into and out of the abdomen tested. 2-3 liters of dialysis fluid is introduced into the abdomen over the next ten to fifteen minutes.[17] The total volume is referred to as a dwell[8] while the fluid itself is referred to as dialysate. The dwell can be as much as 3 liters, and medication can also be added to the fluid immediately before infusion.[17] The dwell remains in the abdomen and waste products diffuse across the peritoneum from the underlying blood vessels. After a variable period of time depending on the treatment (usually 4–6 hours[17] ), the fluid is removed and replaced with fresh fluid. This can occur automatically while the patient is sleeping (automated peritoneal dialysis, APD), or during the day by keeping two litres of fluid in the abdomen at all times, exchanging the fluids four to six times per day (continuous ambulatory peritoneal dialysis, CAPD).[8][18]
The fluid used typically contains sodium chloride, lactate or bicarbonate and a high percentage of glucose to ensure hyperosmolarity. The amount of dialysis that occurs depends on the volume of the dwell, the regularity of the exchange and the concentration of the fluid. APD cycles between 3 and 10 dwells per night, while CAPD involves four dwells per day of 2-3 liters per dwell, with each remaining in the abdomen for 4–8 hours. The viscera accounts for roughly four-fifths of the total surface area of the membrane, but the parietal peritoneum is the most important of the two portions for PD. Two complementary models explain dialysis across the membrane - the three-pore model (in which molecules are exchanged across membranes which sieve molecules, either proteins, electrolytes or water, based on the size of the pores) and the distributed model (which emphasizes the role of capillaries and the solution's ability to increase the number of active capillaries involved in PD). The high concentration of glucose drives the filtration of fluid by osmosis (osmotic UF) from the peritoneal capillaries to the peritoneal cavity. Glucose diffuses rather rapidly from the dialysate to the blood (capillaries). After 4-6 h of the dwell, the glucose osmotic gradient usually becomes too low to allow for further osmotic UF. Therefore, the dialysate will now be reabsorbed from the peritoneal cavity to the capillaries by means of the plasma colloid osmotic pressure, which exceeds the colloid osmotic pressure in the peritoneum by approximately 18-20 mmHg (cf. the Starling mechanism).[19] Lymphatic absorption will also to some extent contribute to the reabsorption of fluid from the peritoneal cavity to the plasma. Patients with a high water permeability (UF-coefficient) of the peritoneal membrane can have an increased reabsorption rate of fluid from the peritoneum by the end of the dwell. The ability to exchange small solutes and fluid in-between the peritoneum and the plasma can be classified as high (fast), low (slow) or intermediate. High transporters tend to diffuse substances well (easily exchanging small molecules between blood and the dialysis fluid, with somewhat improved results with frequent, short-duration dwells such as with APD), while low transporters have a higher UF (due to the slower reabsorption of glucose from the peritoneal cavity, which results in somewhat better results with long-term, high-volume dwells), though in practice either type of transporter can generally be managed through the appropriate use of either APD or CAPD.[20]
Though there are several different shapes and sizes of catheters that can be used, different insertion sites, number of cuffs in the catheter and immobilization, there is no evidence to show any advantages in terms of morbidity, mortality or number of infections, though the quality of information is not yet sufficient to allow for firm conclusions.[21]
A peritoneal equilibration test may be done to assess a person for peritoneal dialysis by determining the characteristics of the peritoneal membrane mass transport characteristics.
Complications
The volume of dialysate removed as well as patient's weight are monitored. If more than 500ml of fluid are retained or a liter of fluid is lost across three consecutive treatments, the patient's physician is generally notified. Excessive loss of fluid can result in hypovolemic shock or hypotension while excessive fluid retention can result in hypertension and edema. Also monitored is the color of the fluid removed: normally it is pink-tinged for the initial four cycles and clear or pale yellow afterward. The presence of pink or bloody effluent suggests bleeding inside the abdomen while feces indicates a perforated bowel and cloudy fluid suggests infection. The patient may also experience pain or discomfort if the dialysate is too acidic, too cold or introduced too quickly, while diffuse pain with cloudy discharge may indicate an infection. Severe pain in the rectum or perineum can be the result of an improperly placed catheter. The dwell can also increase pressure on the diaphragm causing impaired breathing, and constipation can interfere with the ability of fluid to flow through the catheter.[17]
A potentially fatal complication estimated to occur in roughly 2.5% of patients is encapsulating peritoneal sclerosis, in which the bowels become obstructed due to the growth of a thick layer of fibrin within the peritoneum.[22]
The fluid used for dialysis uses glucose as a primary osmotic agent, but this may lead to peritonitis, the decline of kidney and peritoneal membrane function and other negative health outcomes. The acidity, high concentration and presence of lactate and products of the degradation of glucose in the solution (particularly the latter) may contribute to these health issues. Solutions that are neutral, use bicarbonate instead of lactate and have few glucose degradation products may offer more health benefits though this has not yet been studied.[23]
Usage
In a 2004 worldwide survey of patients in end stage renal disease, approximately 11% were receiving PD, compared to the much more common hemodialysis. In Hong Kong and Mexico, PD is more common than the world average, with Mexico conducting most of its dialysis (75%) through PD, while Japan and Germany have rates lower than the world average.[24]
Improvised dialysis
Peritoneal dialysis can be improvised in conditions such as combat surgery or disaster relief using surgical catheters and dialysate made from routinely available medical solutions to provide temporary renal replacement for people with no other options.[25]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 453. hdl:10665/44053. ISBN 9789241547659.
- ↑ Billings, Diane M. (2008-11-01). Lippincott's Content Review for NCLEX-RN. Lippincott Williams & Wilkins. p. 575. ISBN 9781582555157. Archived from the original on 2017-01-13.
- ↑ 3.0 3.1 3.2 3.3 Barkoudah, Ebrahim (2016). "Dialysis Initiation During the Hospital Stay". Volume 5, Issue 4, An Issue of Hospital Medicine Clinics. Elsevier Health Sciences. ISBN 9780323463164. Archived from the original on 2017-04-08.
- ↑ Nolph, K. D. (2013-03-09). "History of peritoneal dialysis". Peritoneal dialysis. Springer Science & Business Media. p. 1.0 and 2.0. ISBN 9789401725606. Archived from the original on 2017-01-13.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Dialysis Sol. Peritoneal W/ Dextrose". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- ↑ 7.0 7.1 Ronco, Claudio; Crepaldi, Carlo; Cruz, Dinna N. (2009). Peritoneal Dialysis: From Basic Concepts to Clinical Excellence. Karger Medical and Scientific Publishers. p. 244. ISBN 9783805592024. Archived from the original on 2017-01-13.
- ↑ 8.0 8.1 8.2 Crowley, LV (2009). An Introduction to Human Disease: Pathology and Pathophysiology Correlations. Jones & Bartlett Publishers. pp. 507–509. ISBN 978-0-7637-6591-0. Archived from the original on 2013-06-18.
- ↑ Ballinger, AE; Palmer, SC; Wiggins, KJ; Craig, JC; Johnson, DW; Cross, NB; Strippoli, GF (26 April 2014). "Treatment for peritoneal dialysis-associated peritonitis" (PDF). The Cochrane Database of Systematic Reviews (4): CD005284. doi:10.1002/14651858.CD005284.pub3. PMID 24771351. Archived (PDF) from the original on 28 August 2021. Retrieved 30 November 2019.
- ↑ Campbell, D; Mudge, DW; Craig, JC; Johnson, DW; Tong, A; Strippoli, GF (8 April 2017). "Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients". The Cochrane Database of Systematic Reviews. 4: CD004679. doi:10.1002/14651858.CD004679.pub3. PMC 6478113. PMID 28390069.
- ↑ Karopadi, AN; Mason G; Rettore E; Ronco C (2013). Zoccali, Carmine (ed.). "Cost of peritoneal dialysis and haemodialysis across the world". Nephrol Dial Transplant. 28 (10): 2553–69. doi:10.1093/ndt/gft214. PMID 23737482.
- ↑ Rabindranath, KS; et al. (2007). Rabindranath, Kannaiyan S (ed.). "Continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis for end-stage renal disease". Cochrane Database of Systematic Reviews. 2 (2): CD006515. doi:10.1002/14651858.CD006515. PMC 6669246. PMID 17443624. Archived from the original on 2009-10-08.
- ↑ Ehrman, JK; Gordon P; Visich PS; Keteyian SJ (2008). Clinical Exercise Physiology. Human Kinetics. pp. 268–269. ISBN 978-0-7360-6565-8. Archived from the original on 2013-06-18.
- ↑ Daly, Conal; Cody, June D.; Khan, Izhar; Rabindranath, Kannaiyan S.; Vale, Luke; Wallace, Sheila A. (2014-08-13). "Double bag or Y-set versus standard transfer systems for continuous ambulatory peritoneal dialysis in end-stage kidney disease". The Cochrane Database of Systematic Reviews (8): CD003078. doi:10.1002/14651858.CD003078.pub2. ISSN 1469-493X. PMC 6457793. PMID 25117423.
- ↑ Wood, M; et al. (2008-08-01). "Nephrology Nursing Standards and Practice Recommendations" (PDF). Canadian Association of Nephrology Nurses and Technologists. Archived from the original (PDF) on 2010-03-31. Retrieved 2010-09-08.
- ↑ Harissis HV, Katsios CS, Koliousi EL, Ikonomou MG, Siamopoulos KC, Fatouros M, Kappas AM (July 2006). "A new simplified one port laparoscopic technique of peritoneal dialysis catheter placement with intra-abdominal fixation". Am. J. Surg. 192 (1): 125–9. doi:10.1016/j.amjsurg.2006.01.033. PMID 16769289.
- ↑ 17.0 17.1 17.2 17.3 Wilkins, Lippincott Williams (2007). Best practices: evidence-based nursing procedures. ISBN 978-1-58255-532-4.
- ↑ McPhee, SJ; Tierney LM; Papadakis MA (2007). Current medical diagnosis and treatment. McGraw-Hill. pp. 934–935. ISBN 978-0-07-147247-0.
{{cite book}}: CS1 maint: url-status (link) - ↑ Rippe B, Venturoli D, Simonsen O, de Arteaga J (2004). "Fluid and electrolyte transport across the peritoneal membrane during CAPD according to the three-pore model". Perit Dial Int. 24 (1): 10–27. doi:10.1177/089686080402400102. PMID 15104333.
- ↑ Daugirdas, JT; Blake PG; Ing TS (2006). "Physiology of Peritoneal Dialysis". Handbook of dialysis. Lippincott Williams & Wilkins. p. 323. ISBN 9780781752534. Archived from the original on 2013-06-18.
- ↑ Htay, Htay; Johnson, David W.; Craig, Jonathan C.; Schena, Francesco Paolo; Strippoli, Giovanni Fm; Tong, Allison; Cho, Yeoungjee (31 May 2019). "Catheter type, placement and insertion techniques for preventing catheter-related infections in chronic peritoneal dialysis patients". The Cochrane Database of Systematic Reviews. 5: CD004680. doi:10.1002/14651858.CD004680.pub3. ISSN 1469-493X. PMC 6543877. PMID 31149735.
- ↑ Kawanishi, H.; Moriishi, M. (2007). "Encapsulating peritoneal sclerosis: prevention and treatment". Peritoneal Dialysis International : Journal of the International Society for Peritoneal Dialysis. 27 Suppl 2: S289–S292. PMID 17556321.
- ↑ Perl, J.; Nessim, S. J.; Bargman, J. M. (2011). "The biocompatibility of neutral pH, low-GDP peritoneal dialysis solutions: Benefit at bench, bedside, or both?". Kidney International. 79 (8): 814–824. doi:10.1038/ki.2010.515. PMID 21248712.
- ↑ Grassmann, A; Gioberge S; Moeller S; Brown G (2005). "ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends". Nephrology Dialysis Transplantation. 20 (12): 2587–2593. doi:10.1093/ndt/gfi159. PMID 16204281. Archived from the original on 2021-08-28. Retrieved 2009-09-30.
- ↑ Pina, J. S.; Moghadam, S.; Cushner, H. M.; Beilman, G. J.; McAlister, V. C. (2010). "In-Theater Peritoneal Dialysis for Combat-Related Renal Failure". The Journal of Trauma: Injury, Infection, and Critical Care. 68 (5): 1253–1256. doi:10.1097/TA.0b013e3181d99089. PMID 20453775. Archived from the original on 2021-08-28. Retrieved 2019-11-30.
External links
- Treatment Methods for Kidney Failure - National Institute of Diabetes and Digestive and Kidney Diseases
| Classification |
|---|