Tyrosinemia
| Tyrosinemia | |
|---|---|
 | |
| Tyrosine | |
Tyrosinemia or tyrosinaemia is an error of metabolism, usually inborn, in which the body cannot effectively break down the amino acid tyrosine. Symptoms of untreated tyrosinemia include liver and kidney disturbances. Without treatment, tyrosinemia leads to liver failure.[1] Today, tyrosinemia is increasingly detected on newborn screening tests before any symptoms appear. With early and lifelong management involving a low-protein diet, special protein formula, and sometimes medication, people with tyrosinemia develop normally, are healthy, and live normal lives.[2]
Signs and symptoms
In terms of the clinical presentation of this condition (and that it presents with 3 types) we find:[3]
- Bleeding tendency
- Ascites
- Enlarged kidney
- Failure to thrive
Cause
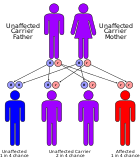
All tyrosinemias result from dysfunction of various genes in the phenylalanine and tyrosine catabolic pathway, and are inherited in an autosomal-recessive pattern.[4]
Type I tyrosinemia results from a mutation in the FAH gene, which encodes the enzyme fumarylacetoacetase.[5] As a result of FAH deficiency, the substrate fumarylacetoacetate can accumulate in proximal renal tubular cells and hepatocytes, resulting in damage to the kidney and liver, respectively.[4]
Type II tyrosinemia results from a mutation in the TAT gene, which encodes the enzyme tyrosine aminotransferase.[5] As a result of TAT deficiency, the substrate tyrosine accumulates, causing ophthalmologic and dermatologic abnormalities.[4]
Type III tyrosinemia results from a mutation in the HPD gene, which encodes the enzyme 4-hydroxyphenylpyruvate dioxygenase.[5] Type III tyrosinemia is the rarest of the three conditions, with only a few cases ever reported.[6] Most of those cases have included intellectual disability and neurologic dysfunction.[4]
Pathophysiology
The mechanism of this condition, Tyrosinemia (its 3 types) finds that mutations in FAH, TAT, or HPD gene cause a decrease in activity of the enzymes responsible for breakdown of tyrosine. This in turn causes toxic levels of tyrosine (and by-products), which in turn can damage several organs including liver, kidneys.[7]

Diagnosis
Types
Type I tyrosinemia can be detected via blood tests for the presence of a fumarylacetoacetate metabolite, succinylacetone, which is considered a pathognomonic indicator for the disease.[8]
Type II tyrosinemia can be detected via the presence of significantly elevated plasma tyrosine levels, and the diagnosis can be confirmed by detection of a mutation in TAT in cultured fibroblasts.[citation needed]
Type III tyrosinemia can be diagnosed by detection of a mutation in HPD in cultured fibroblasts.[4]
Treatment
Treatment varies depending on the specific type; a low-protein diet combined with the use of a specially engineered formula to supply protein is required in most cases. Experience with nitisinone has shown it to be effective. It is a 4-hydroxyphenylpyruvate dioxygenase inhibitor indicated for the treatment of hereditary tyrosinemia type 1 (HT-1) in combination with dietary restriction of tyrosine and phenylalanine.[9] Liver transplant is indicated for patients with tyrosinemia type I who do not respond to nitisinone, as well as those with acute liver failure and hepatomas.[10]
See also
References
- ↑ Shaw K, Bachur R (2016). Fleisher & Ludwig's Textbook of Pediatric Emergency Medicine. Wolters Kluwer. ISBN 978-1451193954.
- ↑ Zea-Rey AV, Cruz-Camino H, Vazquez-Cantu DL, Gutiérrez-García VM, Santos-Guzmán J, Cantú-Reyna C (Nov 2017). "The Incidence of Transient Neonatal Tyrosinemia Within a Mexican Population". Journal of Inborn Errors of Metabolism. 5 (1): 1–4. doi:10.1177/2326409817744230. Archived from the original on 26 August 2021. Retrieved 12 March 2019.
- ↑ "Tyrosinemia type 1 | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 7 December 2021. Retrieved 10 January 2022.
- ↑ 4.0 4.1 4.2 4.3 4.4 Grompe M (2016-12-20). "Disorders of Tyrosine Metabolism". www.uptodate.com. Archived from the original on 2018-02-01. Retrieved 2018-02-23.
- ↑ 5.0 5.1 5.2 Nelson D, Cox M (2013). Lehninger Principles of Biochemistry (6th ed.). New York: WH Freeman and Co. p. 719. ISBN 978-1-4292-3414-6.
- ↑ Heylen E, Scherer G, Vincent MF, Marie S, Fischer J, Nassogne MC (November 2012). "Tyrosinemia Type III detected via neonatal screening: management and outcome". Molecular Genetics and Metabolism. 107 (3): 605–7. doi:10.1016/j.ymgme.2012.09.002. PMID 23036342.
- ↑ "Tyrosinemia: MedlinePlus Genetics". medlineplus.gov. Archived from the original on 28 July 2018. Retrieved 10 January 2022.
- ↑ De Jesús VR, Adam BW, Mandel D, Cuthbert CD, Matern D (2014-09-01). "Succinylacetone as primary marker to detect tyrosinemia type I in newborns and its measurement by newborn screening programs". Molecular Genetics and Metabolism. 113 (1–2): 67–75. doi:10.1016/j.ymgme.2014.07.010. PMC 4533100. PMID 25066104.
- ↑ Swedish Orphan Biovitrum AB, Orfadin [package insert] (PDF), archived (PDF) from the original on 2016-06-24, retrieved 2016-07-12
- ↑ Mieles LA, Esquivel CO, Van Thiel DH, Koneru B, Makowka L, Tzakis AG, Starzl TE (January 1990). "Liver transplantation for tyrosinemia. A review of 10 cases from the University of Pittsburgh". Digestive Diseases and Sciences. 35 (1): 153–7. doi:10.1007/BF01537237. PMC 2974306. PMID 2153069.
External links
| Classification | |
|---|---|
| External resources |
- GeneReview/NCBI/NIH/UW entry on Tyrosinemia Type 1 Archived 2010-06-02 at the Wayback Machine
- Tyrosinemia Archived 2018-07-28 at the Wayback Machine on Genetic Home Reference