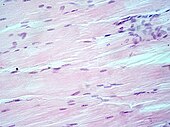
H&E stain

Hematoxylin and eosin stain (or haematoxylin and eosin stain or hematoxylin-eosin stain; often abbreviated as H&E stain or HE stain) is one of the principal tissue stains used in histology.[1][2][3] It is the most widely used stain in medical diagnosis[1] and is often the gold standard.[4] For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.
H&E is the combination of two histological stains: hematoxylin and eosin. The hematoxylin stains cell nuclei a purplish blue, and eosin stains the extracellular matrix and cytoplasm pink, with other structures taking on different shades, hues, and combinations of these colors.[5][6] Hence a pathologist can easily differentiate between the nuclear and cytoplasmic parts of a cell, and additionally, the overall patterns of coloration from the stain show the general layout and distribution of cells and provides a general overview of a tissue sample's structure.[7] Thus, pattern recognition, both by expert humans themselves and by software that aids those experts (in digital pathology), provides histologic information.
This stain combination was introduced in 1877 by chemist Nicolaus Wissozky at the Kasan Imperial University in Russia.[8][7]
Uses
The H&E staining procedure is the principal stain in histology[3][7][2][5] in part because it can be done quickly,[7] is not expensive, and stains tissues in such a way that a considerable amount of microscopic anatomy[9][10] is revealed,[7][5][4] and can be used to diagnose a wide range of histopathologic conditions.[8] The results from H&E staining are not overly dependent on the chemical used to fix the tissue or slight inconsistencies in laboratory protocol,[11] and these factors contribute to its routine use in histology.[7]
H&E staining does not always provide enough contrast to differentiate all tissues, cellular structures, or the distribution of chemical substances,[9] and in these cases more specific stains and methods are used.[10][7]
Method of application

There are many ways to prepare the hematoxylin solutions (formulation) used in the H&E procedure,[11][12][6] in addition, there are many laboratory protocols for producing H&E stained slides,[9] some of which may be specific to a certain laboratory.[7] Although there is no standard procedure,[11][9] the results by convention are reasonably consistent in that cell nuclei are stained blue and the cytoplasm and extracellular matrix are stained pink.[7] Histology laboratories may also adjust the amount or type of staining for a particular pathologist.[7]
After tissues have been collected (often as biopsies) and fixed, they are typically dehydrated and embedded in melted paraffin wax, the resulting block is mounted on a microtome and cut into thin slices.[6] The slices are affixed to microscope slides at which point the wax is removed with a solvent and the tissue slices attached to the slides are rehydrated and are ready for staining.[6] Alternatively, H&E stain is the most used stain in Mohs surgery in which tissues are typically frozen, cut on a cryostat (a microtome that cuts frozen tissue), fixed in alcohol, and then stained.[9]
The H&E staining method involves application of haematoxylin mixed with a metallic salt, or mordant, often followed by a rinse in a weak acid solution to remove excess staining (differentiation), followed by bluing in mildly alkaline water.[13][8][14] After the application of haematoxylin, the tissue is counterstained with eosin (most commonly eosin Y).[6][8][7]
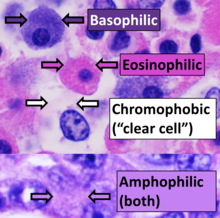
Results
Hematoxylin principally colors the nuclei of cells blue or dark-purple,[6][15][14] along with a few other tissues, such as keratohyalin granules and calcified material. Eosin stains the cytoplasm and some other structures including extracellular matrix such as collagen[5][7][14] in up to five shades of pink.[8] The eosinophilic (substances that are stained by eosin)[5] structures are generally composed of intracellular or extracellular proteins. The Lewy bodies and Mallory bodies are examples of eosinophilic structures. Most of the cytoplasm is eosinophilic and is rendered pink.[10][15] Red blood cells are stained intensely red.[citation needed]
Mode of action
Although hematein, an oxidized form of hematoxylin,[5][16][14] is the active colorant (when combined with a mordant), the stain is still referred to as hematoxylin.[8][13] Hematoxylin, when combined with a mordant (most commonly aluminum alum) is often considered to "resemble"[10] a basic, positively charged, or cationic stain.[5] Eosin is an anionic (negatively charged) and acidic stain.[5][10] The staining of nuclei by hemalum (a combination of aluminum ions and hematein)[14] is ordinarily due to binding of the dye-metal complex to DNA, but nuclear staining can be obtained after extraction of DNA[14] from tissue sections. The mechanism is different from that of nuclear staining by basic (cationic) dyes such as thionine or toluidine blue.[10] Staining by basic dyes occurs only from solutions that are less acidic than hemalum, and it is prevented by prior chemical or enzymatic extraction of nucleic acids. There is evidence to indicate that co-ordinate bonds, similar to those that hold aluminium and hematein together, bind the hemalum complex to DNA and to carboxy groups of proteins in the nuclear chromatin.[citation needed]
The structures do not have to be acidic or basic to be called basophilic and eosinophilic; the terminology is based on the affinity of cellular components for the dyes. Other colors, e.g. yellow and brown, can be present in the sample; they are caused by intrinsic pigments such as melanin. Basal laminae need to be stained by PAS stain or some silver stains, if they have to be well visible. Reticular fibers also require silver stain. Hydrophobic structures also tend to remain clear; these are usually rich in fats, e.g. adipocytes, myelin around neuron axons, and Golgi apparatus membranes.[citation needed]
Examples of H&E stained tissues
-
Bone, cell nuclei (blue-purple), bone matrix (pink).
-
Ductal carcinoma in situ (DCIS) in breast tissue, cell nuclei (blue-purple), extracellular material (pink).
-
Lung tissue taken from an emphysema patient. Cell nuclei (blue-purple), red blood cells (bright red), other cell bodies and extracellular material (pink), and air spaces (white).
-
Muscle tissue, cell nuclei (blue-purple), cell body (pink).
-
Basal cell carcinoma of the skin, cell nuclei (blue-purple), extracellular material (pink).
References
- ^ a b Titford, M. (2005). "The long history of hematoxylin". Biotechnic & Histochemistry. 80 (2): 73–80. doi:10.1080/10520290500138372. PMID 16195172. S2CID 20338201.
- ^ a b Smith C (2006). "Our debt to the logwood tree: the history of hematoxylin". MLO Med Lab Obs. 38 (5): 18, 20–2. PMID 16761865.
- ^ a b Dapson RW, Horobin RW (2009). "Dyes from a twenty-first century perspective". Biotech Histochem. 84 (4): 135–7. doi:10.1080/10520290902908802. PMID 19384743. S2CID 28563610.
- ^ a b Rosai J (2007). "Why microscopy will remain a cornerstone of surgical pathology". Lab Invest. 87 (5): 403–8. doi:10.1038/labinvest.3700551. PMID 17401434.
- ^ a b c d e f g h Chan JK (2014). "The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology". Int J Surg Pathol. 22 (1): 12–32. doi:10.1177/1066896913517939. PMID 24406626. S2CID 26847314.
- ^ a b c d e f Stevens, Alan (1982). "The Haematoxylins". In Bancroft, John; Stevens, Alan (eds.). The Theory and Practice of Histological Techniques (2nd ed.). Longman Group Limited. p. 109.
- ^ a b c d e f g h i j k l Wittekind D (2003). "Traditional staining for routine diagnostic pathology including the role of tannic acid. 1. Value and limitations of the hematoxylin-eosin stain". Biotech Histochem. 78 (5): 261–70. doi:10.1080/10520290310001633725. PMID 14989644. S2CID 10563849.
- ^ a b c d e f Titford, Michael (2009). "Progress in the Development of Microscopical Techniques for Diagnostic Pathology". Journal of Histotechnology. 32 (1): 9–19. doi:10.1179/his.2009.32.1.9. ISSN 0147-8885. S2CID 26801839.
- ^ a b c d e Larson K, Ho HH, Anumolu PL, Chen TM (2011). "Hematoxylin and eosin tissue stain in Mohs micrographic surgery: a review". Dermatol Surg. 37 (8): 1089–99. doi:10.1111/j.1524-4725.2011.02051.x. PMID 21635628. S2CID 2538853.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Ross, Michael H.; Pawlina, Wojciech (2016). Histology : a text and atlas : with correlated cell and molecular biology (7th ed.). Wolters Kluwer. pp. 984p. ISBN 978-1451187427.
- ^ a b c Schulte EK (1991). "Standardization of biological dyes and stains: pitfalls and possibilities". Histochemistry. 95 (4): 319–28. doi:10.1007/BF00266958. PMID 1708749. S2CID 29628388.
- ^ Llewellyn BD (2009). "Nuclear staining with alum hematoxylin". Biotech Histochem. 84 (4): 159–77. doi:10.1080/10520290903052899. PMID 19579146. S2CID 205713596.
- ^ a b Ortiz-Hidalgo C, Pina-Oviedo S (2019). "Hematoxylin: Mesoamerica's Gift to Histopathology. Palo de Campeche (Logwood Tree), Pirates' Most Desired Treasure, and Irreplaceable Tissue Stain". Int J Surg Pathol. 27 (1): 4–14. doi:10.1177/1066896918787652. PMID 30001639.
- ^ a b c d e f Kiernan JA (2018). "Does progressive nuclear staining with hemalum (alum hematoxylin) involve DNA, and what is the nature of the dye-chromatin complex?". Biotech Histochem. 93 (2): 133–148. doi:10.1080/10520295.2017.1399466. PMID 29320873. S2CID 13481905.
- ^ a b Leeson, Thomas S.; Leeson, C. Roland (1981). Histology (Fourth ed.). W. B. Saunders Company. p. 600. ISBN 978-0721657042.
- ^ Kahr, Bart; Lovell, Scott; Subramony, Anand (1998). "The progress of logwood extract". Chirality. 10 (1–2): 66–77. doi:10.1002/chir.12.
Further reading
- Kiernan JA (2008) Histological and Histochemical Methods: Theory and Practice. 4th ed. Bloxham, UK: Scion.
- Lillie RD, Pizzolato P, Donaldson PT (1976) Nuclear stains with soluble metachrome mordant lake dyes. The effect of chemical endgroup blocking reactions and the artificial introduction of acid groups into tissues. Histochemistry 49: 23–35.
- Llewellyn BD (2009) Nuclear staining with alum-hematoxylin. Biotech. Histochem. 84: 159–177.
- Puchtler H, Meloan SN, Waldrop FS (1986) Application of current chemical concepts to metal-haematein and -brazilein stains. Histochemistry 85: 353–364.
External links
Protocol
- CS1 maint: multiple names: authors list
- Articles with short description
- Short description is different from Wikidata
- All articles with unsourced statements
- Articles with unsourced statements from April 2023
- Commons category link from Wikidata
- Microscopy
- Microbiology techniques
- Laboratory techniques
- Histopathology
- Histotechnology
- Staining dyes
- Staining