Gallium (68Ga) gozetotide
 | |
 | |
| Names | |
|---|---|
| Trade names | Illuccix, Locametz |
| Other names | Gallium 68 PSMA-11, Gallium Ga 68 gozetotide (USAN US) |
| |
| Clinical data | |
| Drug class | Radioactive diagnostic agent[1] |
| Main uses | Positron emission tomography (PET) of prostate cancer[1] |
| Side effects | Nausea, diarrhea, dizziness[1] |
| Pregnancy category |
|
| Routes of use | Intravenous[1] |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Excretion | Urine[1] |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
Gallium (68Ga) gozetotide, sold under the brand name Illuccix, is a radioactive diagnostic agent used for imaging prostate cancer by positron emission tomography (PET).[1] Specifically it is used in cases that are prostate-specific membrane antigen (PSMA) positive.[1] It is given by injection into a vein.[1]
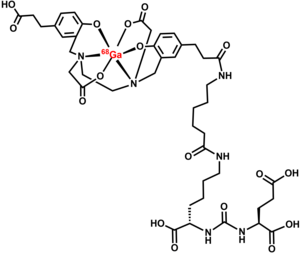
Common side effects include nausea, diarrhea, and dizziness.[1] Other side effects include radiation exposure.[1] It is made of 68Ga attached to the prostate-specific membrane antigen (PSMA) targeting ligand, Glu-Urea-Lys(Ahx).[6]
Gallium (68Ga) gozetotide was approved for medical use in the United States in 2020.[1] As of 2022 it has been recommended for approval, but has not yet been approved in Europe.[7] In the United Kingdom it costs the NHS about £2,000 per 25 ucg as of 2022.[7]
Medical uses
Gallium (68Ga) gozetotide is a radioactive diagnostic agent indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA)-positive lesions in men with prostate cancer.[4][5]
Ga 68 PSMA-11 injections are used for PET imaging of prostate-specific membrane antigen (PSMA) positive lesions in males with prostate cancer. It can be given for the patients with suspected metastasis, and the candidates with initial definitive therapy.[1]
Dosage
It is given at a dose of 111 MBq to 259 MBq (3 mCi to 7 mCi) about 50 to 100 minutes before imaging.[1]
Mechanism of action
Gallium (68Ga) gozetotide binds with prostate-specific membrane antigen (PSMA).[1] This binds to cells that express PSMA, including malignant prostate cancer cells.[1] The radioactive isotope of gallium, 68Ga is responsible for emitting β+ radiations and X-rays.[1] This helps in recording images by positron emission tomography (PET) and CT scan.[1]
History
Initially gallium (68Ga) chloride solution injections used for radiolabelling,[8] in 2019 European Pharmacopoeia mentions gallium (68Ga) DOTATOC injection for radiolabelling and PET imaging.[9]
Ga 68 PSMA-11 is co-developed by University of California, Los Angeles and University of California, San Francisco, they conducted phase III clinical trial.[10] In December 2020, the drug was first approved by US Food and Drug Administration (FDA) for PET imaging.[11] This is the first drug approved by the US Food and Drug Administration (FDA) as a PET imaging agent.[11]
Society and culture
Legal status
On 13 October 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Locametz, intended for the diagnosis of prostate cancer.[12] The applicant for this medicinal product is Novartis Europharm Limited.[12]
Names
Gallium (68Ga) gozetotide is the international nonproprietary name (INN).[13]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 "Gallium GA-68 PSMA-11- gallium ga-68 gozetotide injection, solution". DailyMed. 21 November 2021. Archived from the original on 3 July 2022. Retrieved 27 May 2022.
- ↑ 2.0 2.1 "AusPAR: Glu-urea-Lys(ahx)-hbed-CC". Therapeutic Goods Administration (TGA). 27 June 2022. Retrieved 17 July 2022.
{{cite web}}: CS1 maint: url-status (link) - ↑ "Gallium GA-68 PSMA-11- gallium ga-68 gozetotide injection, solution". DailyMed. 30 November 2021. Archived from the original on 3 July 2022. Retrieved 27 May 2022.
- ↑ 4.0 4.1 "Illuccix- kit for the preparation of gallium ga 68 gozetotide injection kit". DailyMed. 12 May 2022. Archived from the original on 3 July 2022. Retrieved 27 May 2022.
- ↑ 5.0 5.1 "Locametz- kit for the preparation of gallium ga 68 gozetotide injection, powder, lyophilized, for solution". DailyMed. 23 March 2022. Archived from the original on 3 July 2022. Retrieved 27 May 2022.
- ↑ "https://www.cancer.gov/publications/dictionaries/cancer-drug/def/gallium-ga-68-gozetotide?redirect=true". www.cancer.gov. 2 February 2011. Archived from the original on 18 November 2021. Retrieved 3 November 2022.
{{cite web}}: External link in|title= - ↑ 7.0 7.1 "Gozetotide". SPS - Specialist Pharmacy Service. 16 August 2022. Archived from the original on 3 November 2022. Retrieved 3 November 2022.
- ↑ "Gallium (68Ga) Chloride Solution for Radiolabelling". European Pharmacopoeia (9th ed.). Stuttgart. 2018. p. 1148. ISBN 978-3-7692-6816-4.
- ↑ "Gallium (68Ga) DOTATOC injection". European Pharmacopoeia (10th ed.). Stuttgart. 2019. p. 1208. ISBN 978-3-7692-7453-0.
- ↑ Carlucci G, Ippisch R, Slavik R, Mishoe A, Blecha J, Zhu S (February 2021). "68Ga-PSMA-11 NDA Approval: A Novel and Successful Academic Partnership". Journal of Nuclear Medicine. 62 (2): 149–155. doi:10.2967/jnumed.120.260455. PMC 8679592. PMID 33443068.
- ↑ 11.0 11.1 "FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer". U.S. Food and Drug Administration (FDA) (Press release). 2 December 2020. Archived from the original on 4 November 2021. Retrieved 9 November 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 12.0 12.1 "Locametz: Pending EC decision". European Medicines Agency. 13 October 2022. Archived from the original on 14 October 2022. Retrieved 14 October 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ World Health Organization (2021). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 85" (PDF). WHO Drug Information. 35 (1). Archived (PDF) from the original on 19 April 2021. Retrieved 28 May 2022.
External links
| Identifiers: |
|
|---|
- "Gallium Ga 68 gozetotide". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 1 November 2022. Retrieved 14 October 2022.
- Pages using duplicate arguments in template calls
- CS1 maint: url-status
- CS1 errors: external links
- Wikipedia articles incorporating the PD-notice template
- Use dmy dates from June 2022
- Articles with invalid date parameter in template
- Infobox drug with local INN variant
- Drugs with non-standard pregnancy category
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Gallium compounds
- Radiopharmaceuticals
- RTT