Piflufolastat F-18
 | |
| Names | |
|---|---|
| Trade names | Pylarify |
| Other names | 18F-DCFPyL |
| Clinical data | |
| Drug class | Radiopharmaceutical[1] |
| Main uses | Prostate cancer positron emission tomography (PET) imaging[2] |
| Side effects | Headache, altered taste, tiredness[2] |
| Routes of use | Intravenous |
| Typical dose | 333 MBq (9 mCi)[2] |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
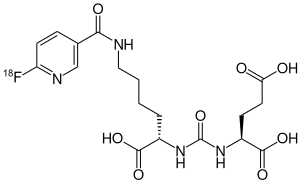
| Formula | C18H23[18F]N4O8 |
| Molar mass | 441.4 g/mol |
| 3D model (JSmol) | |
| |
| |
Piflufolastat F-18, sold under the brand name Pylarify, is a radioactive diagnostic agent used in positron emission tomography (PET) imaging.[2] Specifically it is used to image prostate cancer that is prostate-specific membrane antigen (PSMA) positive to look for spread or recurrence.[2] It is given by injection into a vein.[2]
Common side effects include headache, altered taste, and tiredness.[2] Other side effects may include allergic reactions and radiation exposure.[2]
Piflufolastat F-18 was approved for medical use in the United States in 2021.[2] It is not approved in either Europe or the United Kingdom as of 2022.[1]
Medical uses
Piflufolastat F-18 is indicated for people with suspected prostate cancer metastasis (when cancer cells spread from the place where they first formed to another part of the body) who are potentially curable by surgery or other therapy.[2][3] Piflufolastat F-18 is also indicated for people with suspected prostate cancer recurrence based on elevated serum prostate-specific antigen (PSA) levels.[2][3]
Dosage
The typical dose is 333 MBq (9 mCi) 60 min before imaging.[2]
History
The safety and efficacy of piflufolastat F-18 were evaluated in two prospective clinical trials with a total of 593 men with prostate cancer who each received one injection of piflufolastat F-18.[3] In the first trial, a cohort of 268 participants with biopsy-proven prostate cancer underwent PET/CT scans performed with piflufolastat F-18.[3] These participants were candidates for surgical removal of the prostate gland and pelvic lymph nodes and were considered at higher risk for metastasis.[3] Among the participants who proceeded to surgery, those with positive readings in the pelvic lymph nodes on piflufolastat F-18 PET had a clinically important rate of metastatic cancer confirmed by surgical pathology.[3]
The second trial enrolled 208 participants who had rising serum PSA levels after initial prostate surgery or other definitive therapy, and thus had biochemical evidence of recurrent prostate cancer.[3] Prior to a single piflufolastat F-18 PET/CT scan, all of these participants had baseline conventional imaging performed that did not show definite spread of prostate cancer.[3] Piflufolastat F-18 PET detected at least one positive lesion in at least one body region (bone, prostate bed, pelvic lymph node, other lymph nodes, or soft tissue) in 60% of these participants.[3] In participants with positive piflufolastat F-18 PET readings who had correlative tissue pathology from biopsies, results from baseline or follow-up imaging by conventional methods, or serial PSA levels available for comparison, local recurrence or metastasis of prostate cancer was confirmed in an estimated 85% to 87% of cases, depending on the reader.[3] Thus, the second trial demonstrated that piflufolastat F-18 PET can detect sites of disease in participants with biochemical evidence of recurrent prostate cancer, thereby providing important information that may impact the approach to therapy.[3]
The U.S. Food and Drug Administration (FDA) granted approval of Pylarify to Progenics Pharmaceuticals, Inc.[3] It is the second PSMA-targeted PET imaging drug approved by the U.S. Food and Drug Administration (FDA).[3] The first approved PSMA-targeted PET imaging drug is Ga 68 PSMA-11.[3]
References
- ↑ 1.0 1.1 "Fluorine-18-DCFPyL". SPS - Specialist Pharmacy Service. 26 December 2019. Retrieved 29 October 2022.
{{cite web}}: CS1 maint: url-status (link) - ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "Pylarify- piflufolastat f-18 injection". DailyMed. Archived from the original on 13 September 2021. Retrieved 12 September 2021.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 "FDA approves PSMA-targeted imaging drug for men with prostate cancer". U.S. Food and Drug Administration (FDA). 27 May 2021. Archived from the original on 11 September 2021. Retrieved 12 September 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
![]() This article incorporates public domain material from the United States Department of Health and Human Services website http://www.fda.gov.
This article incorporates public domain material from the United States Department of Health and Human Services website http://www.fda.gov.
External links
| Identifiers: |
|
|---|
- "Piflufolastat F18". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 18 February 2022. Retrieved 1 September 2022.
- "SDS Data Sheet" (PDF). Archived (PDF) from the original on 30 April 2022. Retrieved 1 September 2022.
- Clinical trial number NCT02981368 for "Study of 18F-DCFPyL PET/CT Imaging in Patients With Prostate Cancer (OSPREY)" at ClinicalTrials.gov
- Clinical trial number NCT03739684 for "Study of 18F-DCFPyL PET/CT Imaging in Patients With Suspected Recurrence of Prostate Cancer (CONDOR)" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- CS1 maint: url-status
- Wikipedia articles incorporating the PD-notice template
- Use American English from September 2021
- Articles with invalid date parameter in template
- All Wikipedia articles written in American English
- Use dmy dates from September 2021
- Infobox-drug molecular-weight unexpected-character
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Wikipedia articles incorporating text from the United States Department of Health and Human Services
- Drugs not assigned an ATC code
- Medicinal radiochemistry
- PET radiotracers
- Radiopharmaceuticals
- RTT
- All stub articles
- Pharmacology stubs