Copper (64Cu) oxodotreotide
 | |
| Names | |
|---|---|
| Trade names | Detectnet |
| Other names | Copper Cu 64 dotatate |
| |
| Clinical data | |
| Drug class | Radioactive diagnostic agent[1] |
| Main uses | Positron emission tomography (PET) of neuroendocrine tumors (NETs)[1] |
| Side effects | Nausea, vomiting, flushing[1] |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
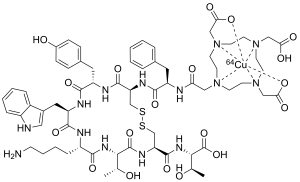
| Formula | C65H88CuN14O19S2 |
| Molar mass | 1497.16 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Copper (64Cu) oxodotreotide, sold under the brand name Detectnet, is a radioactive diagnostic agent used in positron emission tomography (PET) of neuroendocrine tumors (NETs).[1] Specifically it is used in adults with somatostatin receptor positive disease.[1] It is given by injection into a vein.[1]
Common side effects include nausea, vomiting, and flushing.[1] Other side effects include radiation exposure and allergic reactions.[1] It interacts with somatostatin analogs.[1] Use during pregnancy may harm the baby.[1] It works by binding to somatostatin receptor, particularly subtype 2 receptors.[1]
Copper (64Cu) oxodotreotide was approved for medical use in the United States in 2020.[1] In the United States it costs about 3,900 USD for a vial as of 2022.[2]
Medical uses
Dosage
It is typically used at a dose of 148 MBq (4 mCi).[1]
History
The U.S. Food and Drug Administration (FDA) approved copper 64Cu dotatate based on data from two trials that evaluated 175 adults.[3]
Trial 1 evaluated adults, some of whom had known or suspected NETs and some of whom were healthy volunteers.[3] The trial was conducted at one site in the United States (Houston, TX).[3] Both groups received copper 64Cu dotatate and underwent PET scan imaging.[3]
Trial 2 data came from the literature-reported trial of 112 adults, all of whom had history of NETs and underwent PET scan imaging with copper 64Cu dotatate.[3] The trial was conducted at one site in Denmark.[3] In both trials, copper 64Cu dotatate images were compared to either biopsy results or other images taken by different techniques to detect the sites of a tumor.[3] The images were read as either positive or negative for presence of NETs by three independent image readers who did not know participant clinical information.[3]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Detectnet- copper cu 64 dotatate injection, solution". DailyMed. 14 September 2020. Archived from the original on 1 November 2022. Retrieved 24 September 2020.
- ↑ "Detectnet Prices, Coupons, Copay & Patient Assistance". Drugs.com. Archived from the original on 22 May 2022. Retrieved 3 November 2022.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Drug Trials Snapshots: Detectnet". U.S. Food and Drug Administration (FDA). 3 September 2020. Archived from the original on 21 September 2020. Retrieved 10 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
| Identifiers: |
|---|
- "Copper dotatate Cu-64". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2022-05-21. Retrieved 2022-10-20.
- "copper Cu 64 dotatate injection safety data sheet" (PDF). Curium US LLC. 15 March 2020. Archived (PDF) from the original on 27 October 2020. Retrieved 20 October 2022.
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs not assigned an ATC code
- Radiopharmaceuticals
- Orphan drugs
- DOTA (chelator) derivatives
- Copper complexes
- RTT
- All stub articles
- Pharmacology stubs