Coal tar
| Names | |
|---|---|
| Trade names | Balnetar, Cutar, others |
| Other names | liquor carbonis detergens (LCD) liquor picis carbonis (LPC)[1] |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | Topical |
| Defined daily dose | not established[2] |
| External links | |
| AHFS/Drugs.com | Multum Consumer Information |
| Legal | |
| Legal status |
|
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal.[3][4] It has both medical and industrial uses.[3][5] It may be applied to the affected area to treat psoriasis and seborrheic dermatitis (dandruff).[6] It may be used in combination with ultraviolet light therapy.[6] Industrially it is a railway tie preservative and used in the surfacing of roads.[7]
Side effects include skin irritation, sun sensitivity, allergic reactions, and skin discoloration.[6] It is unclear if use during pregnancy is safe for the baby and use during breastfeeding is not typically recommended.[8] The exact mechanism of action is unknown.[9] It is a complex mixture of phenols, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic compounds.[3] It demonstrates antifungal, anti-inflammatory, anti-itch, and antiparasitic properties.[9]
Coal tar was discovered around 1665 and used for medical purposes as early as the 1800s.[7][10] It is on the World Health Organization's List of Essential Medicines.[11] Coal tar is available as a generic medication and over the counter.[5] In the United Kingdom 125 ml of 5% shampoo costs the NHS about £1.89.[12] In the United States a month's treatment costs less than $25 USD.[5] Coal tar was one of the key starting materials for the early pharmaceutical industry.[13]
Uses
Medicine
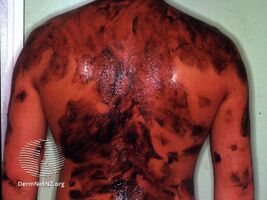
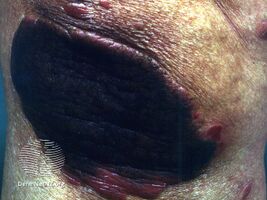
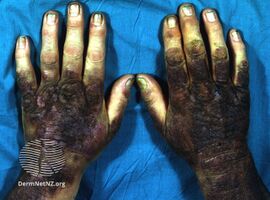
Coal tar is used in medicated shampoo, soap and ointment. It demonstrates antifungal, anti-inflammatory, anti-itch, and antiparasitic properties.[9] It may be applied topically as a treatment for dandruff and psoriasis, and to kill and repel head lice.[6] It may be used in combination with ultraviolet light therapy.[6]
Pine tar has historically also been used for this purpose. Though it is frequently cited online as having been banned as a medical product by the FDA due to a "lack of evidence having been submitted for proof of effectiveness", pine tar is included in the Code of Federal Regulations, subchapter D: Drugs for Human Use, as an OTC treatment for "Dandruff/seborrheic dermatitis/psoriasis".[14]
Coal tar may be used in two forms: crude coal tar (Latin: pix carbonis) or a coal tar solution (Latin: liquor picis carbonis, LPC) also known as liquor carbonis detergens (LCD).[9][15][16] Named brands include Denorex, Balnetar, Psoriasin, Tegrin, T/Gel, and Neutar. When used in the extemporaneous preparation of topical medications, it is supplied in the form of coal tar topical solution USP, which consists of a 20% w/v solution of coal tar in alcohol, with an additional 5% w/v of polysorbate 80 USP; this must then be diluted in an ointment base such as petrolatum.
-
Coal tar/treatment
-
Coal tar/treatment
-
Coal tar/treatment
Dosage
The defined daily dose is not established.[2]
Construction
Coal tar was a component of the first sealed roads. In its original development by Edgar Purnell Hooley, tarmac was tar covered with granite chips. Later the filler used was industrial slag. Today, petroleum derived binders and sealers are more commonly used. These sealers are used to extend the life and reduce maintenance cost associated with asphalt pavements, primarily in asphalt road paving, car parks and walkways.
Coal tar is incorporated into some parking-lot sealcoat products used to protect the structural integrity of the underlying pavement.[17] Sealcoat products that are coal-tar based typically contain 20 to 35 percent coal-tar pitch.[17] Research[18] shows it is used throughout the United States of America, however several areas have banned its use in sealcoat products, [19][20][21] including the District of Columbia; the city of Austin, Texas; Dane County, Wisconsin; the state of Washington; and several municipalities in Minnesota and others.[22][23]
Industry
Being flammable, coal tar is sometimes used for heating or to fire boilers. Like most heavy oils, it must be heated before it will flow easily.
A large part of the binder used in the graphite industry for making "green blocks" is coke oven volatiles (COV), a considerable portion of which is coal tar. During the baking process of the green blocks as a part of commercial graphite production, most of the coal tar binders are vaporised and are generally burned in an incinerator to prevent release into the atmosphere, as COV and coal tar can be injurious to health.
Coal tar is also used to manufacture paints, synthetic dyes (notably tartrazine/Yellow #5), and photographic materials.
In the coal gas era, there were many companies in Britain whose business was to distill coal tar to separate the higher-value fractions, such as naphtha, creosote and pitch. A great many industrial chemicals were first isolated from coal tar during this time. These companies included:[24]
- British Tar Products
- Lancashire Tar Distillers
- Midland Tar Distillers
- Newton, Chambers & Company (owners of Izal brand disinfectant)
- Sadlers Chemicals
Safety
Side effects of coal tar products include skin irritation, sun sensitivity, allergic reactions, and skin discoloration.[6] It is unclear if use during pregnancy is safe for the baby and use during breastfeeding is not typically recommended.[25]
According to the National Psoriasis Foundation, coal tar is a valuable, safe and inexpensive treatment option for millions of people with psoriasis and other scalp or skin conditions.[26] According to the FDA, coal tar concentrations between 0.5% and 5% are considered safe[27] and effective for psoriasis.
Cancer
Evidence is inconclusive whether the coal tar in the concentrations seen in non-prescription treatments causes cancer, because there is insufficient data to make a judgment.[28] While coal tar consistently causes cancer in animal studies,[29] short-term treatments of humans have shown no significant increase in rates of cancer.[28] It's possible that the skin can repair itself after short-term exposure to PAHs, but not after long-term exposure.[28]
Coal tar was one of the first chemical substances proven to cause cancer from occupational exposure, during research in 1775 on the cause of chimney sweeps' carcinoma.[30] Modern studies have shown that working with coal tar pitch, such as during the paving of roads or when working on roofs, increases the risk of cancer.[29]
Coal tar contains many polycyclic aromatic hydrocarbons, and it is believed that their metabolites bind to DNA, damaging it.[31] Long-term skin exposure to these compounds can produce "tar warts", which can progress to squamous cell carcinoma.[30]
The International Agency for Research on Cancer lists coal tars as Group 1 carcinogens, meaning they directly cause cancer.[29][32][33] Both the U.S. Department of Health and Human Services and the state of California list coal tars as known human carcinogens.[34]
In response to public health concerns regarding the carcinogenicity of PAHs some municipalities, such as the city of Milwaukee, have banned the use of common coal tar-based road and driveway sealants citing concerns of elevated PAH content in groundwater.[35]
Other
Coal tar causes increased sensitivity to sunlight,[36] so skin treated with topical coal tar preparations should be protected from sunlight.
The residue from the distillation of high-temperature coal tar, primarily a complex mixture of three or more membered condensed ring aromatic hydrocarbons, was listed on 28 October 2008 as a substance of very high concern by the European Chemicals Agency.
Mechanism of action
The exact mechanism of action is unknown.[9] Coal tar is a complex mixture of phenols, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic compounds.[3]
It is a keratolytic agent, which reduces the growth rate of skin cells and softens the skin's keratin.[37][30]
Composition
Coal tar is produced through thermal destruction (pyrolysis) of coal. Its composition varies with the process and type of coal used – lignite, bituminous or anthracite.[30]
Coal tar contains approximately 10,000 chemicals, of which only about 50% have been identified.[38][better source needed] Components include polycyclic aromatic hydrocarbons (4-rings: chrysene, fluoranthene, pyrene, triphenylene, naphthacene, benzanthracene, 5-rings: picene, benzo[a]pyrene, benzo[e]pyrene, benzofluoranthenes, perylene, 6-rings: dibenzopyrenes, dibenzofluoranthenes, benzoperylenes, 7-rings: coronene), as well as methylated and polymethylated derivatives, mono- and polyhydroxylated derivatives, and heterocyclic compounds.[29][39] Others include benzene, toluene, xylenes, cumenes, coumarone, indene, benzofuran, naphthalene and methyl-naphthalenes, acenaphthene, fluorene, phenol, cresols, pyridine, picolines, phenanthracene, carbazole, quinolines, fluoranthene.[30] Many of these constituents are known carcinogens.[40][31]
Derivatives
Various phenolic coal tar derivatives have analgesic (pain-killer) properties. These included acetanilide, phenacetin, and paracetamol (acetaminophen).[41] Paracetamol is the only coal-tar derived analgesic still in use today, but industrial phenol is now usually synthesized from crude oil rather than coal tar. Coal tar derivatives are contra-indicated for people with the inherited red cell blood disorder glucose-6-phosphate dehydrogenase deficiency (G6PD deficiency), as they can cause oxidative stress leading to red blood cell breakdown.[42]
Society and culture
Coal tar is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[11] Coal tar is generally available as a generic medication and over the counter.[5] In the United Kingdom 125 ml of 5% shampoo costs the NHS about £1.89.[12] In the United States a month of treatment costs less than US$25.[5]
Regulation
Exposure to coal tar pitch volatiles can occur in the workplace by breathing, skin contact, or eye contact. The Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit) to 0.2 mg/m3 benzene-soluble fraction over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 0.1 mg/m3 cyclohexane-extractable fraction over an 8-hour workday. At levels of 80 mg/m3, coal tar pitch volatiles are immediately dangerous to life and health.[43]
When used as a medication in the United States, coal tar preparations are considered over-the-counter drug pharmaceuticals and are subject to regulation by the Food and Drug Administration (FDA).
See also
References
- ↑ Berenblum I (25 September 1948). "Liquor Picis Carbonis". British Medical Journal. 2 (4577): 601. doi:10.1136/bmj.2.4577.601. PMC 2091540. PMID 18882998.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2021. Retrieved 13 September 2020.
- ↑ 3.0 3.1 3.2 3.3 "Background and Environmental Exposures to Creosote in the United States" (PDF). cdc.gov. September 2002. p. 19. Archived (PDF) from the original on 25 January 2017. Retrieved 13 January 2017.
- ↑ Vallee, Yannick (1998). Gas Phase Reactions in Organic Synthesis. CRC Press. p. 107. ISBN 9789056990817. Archived from the original on 2017-09-18. Retrieved 2017-09-09.
- ↑ 5.0 5.1 5.2 5.3 5.4 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. X. ISBN 9781284057560.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 308. hdl:10665/44053. ISBN 9789241547659.
- ↑ 7.0 7.1 Hornbostel, Caleb (1991). Construction Materials: Types, Uses and Applications. John Wiley & Sons. p. 864. ISBN 9780471851455. Archived from the original on 2017-09-18.
- ↑ "Coal Tar use while Breastfeeding | Drugs.com". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 13 January 2017.
- ↑ 9.0 9.1 9.2 9.3 9.4 Maibach, Howard I. (2011). Evidence Based Dermatology. PMPH-USA. pp. 935–936. ISBN 9781607950394. Archived from the original on 2017-09-18.
- ↑ Sneader, Walter (2005). Drug Discovery: A History. John Wiley & Sons. p. 356. ISBN 9780471899792. Archived from the original on 2017-09-18.
- ↑ 11.0 11.1 World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 12.0 12.1 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 829. ISBN 9780857111562.
- ↑ Ravina, Enrique (2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 23. ISBN 9783527326693. Archived from the original on 2017-09-18.
- ↑ "Title 21 – Food and Drugs. CHAPTER I – FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES (CONTINUED): SUBCHAPTER D—DRUGS FOR HUMAN USE". United States Government Publishing Office. 1 March 2008. Archived from the original on 2016-03-10. Retrieved 3 March 2016.
- ↑ Hughes, Jeff; Donnelly, Richard; James-Chatgilaou, Greta (2001). Clinical pharmacy : a practical approach - Society of Hospital Pharmacists of Australia. South Yarra: Macmillan Publishers Australia. p. 114. ISBN 9780732980290.
- ↑ Paghdal KV; Schwartz RA (31 January 2009). "Topical tar: back to the future". J Am Acad Dermatol. 61 (2): 294–302. doi:10.1016/j.jaad.2008.11.024. PMID 19185953.
- ↑ 17.0 17.1 Mahler BJ; Van Metre PC (2 February 2011). "Coal-Tar-Based Pavement Sealcoat, Polycyclic Aromatic Hydrocarbons (PAHs), and Environmental Health". U.S. Geological Survey Fact Sheet. Archived from the original on 2013-03-28. Retrieved 8 March 2013.
- ↑ Van Metre PC; Mahler BJ (15 December 2010). "Contribution of PAHs from coal-tar pavement sealcoat and other sources to 40 U.S. lakes". U.S. Geological Survey. 409 (2): 334–44. Bibcode:2010ScTEn.409..334V. doi:10.1016/j.scitotenv.2010.08.014. PMID 21112613.
- ↑ "City of Austin Ordinance 20051117-070" (PDF). 17 November 2005. Archived (PDF) from the original on 2013-05-31. Retrieved 8 March 2013.
- ↑ "District Bans Coal-Tar Pavement Products". 26 June 2009. Archived from the original on 2012-12-26. Retrieved 8 March 2013.
- ↑ "Ordinance 80 : Establishing Regulations on Coal Tar Sealcoat Products Application and Sale" (PDF). Dane County Office of Lakes and Watersheds. 1 July 2007. Archived (PDF) from the original on 2011-08-24. Retrieved 8 March 2013.
- ↑ "Coal Tar Free America – Bans". Archived from the original on 2014-10-06. Retrieved 8 March 2013.
- ↑ Barbara J Mahler (14 April 2011). Causes of Increasing Concentrations of Polycyclic Aromatic Hydrocarbons (PAHs) in U.S. Lakes (PDF). PAHs Increasing in Urban U.S. Lakes. Environmental and Energy Study Institute. Archived from the original (PDF) on 5 October 2011. Retrieved 8 March 2013.
{{cite conference}}: Unknown parameter|conferenceurl=ignored (help) - ↑ Mike Smith. "GANSG – Coal Tar Distillers". Igg.org.uk. Archived from the original on 2013-06-19. Retrieved 8 March 2013.
- ↑ "Coal Tar use while Breastfeeding | Drugs.com". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 13 January 2017.
- ↑ "The battle to save coal tar in California". 3 December 2001. Archived from the original on 2002-10-29. Retrieved 8 March 2013.
- ↑ FDA (1 April 2015). "Drug Products for the Control of Dandruff, Seborrheic Dermatitis, and Psoriasis". Archived from the original on September 18, 2015. Retrieved 26 February 2016.
- ↑ 28.0 28.1 28.2 Roelofzen, Judith H. J.; Aben, Katja K. H.; Oldenhof, Ursula T. H.; Coenraads, Pieter-Jan; Alkemade, Hans A.; Kerkhof, Peter C. M. van de; Valk, Pieter G. M. van der; Kiemeney, Lambertus A. L. M. (2010-04-01). "No Increased Risk of Cancer after Coal Tar Treatment in Patients with Psoriasis or Eczema" (PDF). Journal of Investigative Dermatology. 130 (4): 953–961. doi:10.1038/jid.2009.389. ISSN 0022-202X. PMID 20016499. Archived (PDF) from the original on 2020-07-28. Retrieved 2020-03-17.
- ↑ 29.0 29.1 29.2 29.3 "Coal-tar pitch" (PDF). IARC. IARC. Archived (PDF) from the original on 21 May 2016. Retrieved 10 June 2017.
it was concluded that there is sufficient evidence in humans for the carcinogenicity of occupational exposures during paving and roofing with coal tar pitch. ... Six coal-tar pitches and three extracts of coal-tar pitches all produced skin tumours, including carcinomas, when applied to the skin of mice
- ↑ 30.0 30.1 30.2 30.3 30.4 Roberts, L. (2014). "Coal Tar". In Wexler, Philip (ed.). Encyclopedia of Toxicology (Third Edition). Oxford: Academic Press. pp. 993–995. doi:10.1016/b978-0-12-386454-3.00012-9. ISBN 9780123864550.
composition of coal tar will be influenced by the process used for pyrolytic distillation as well as by the original composition of the coal ... He then demonstrated excess cancers occurring in laboratory animals when coal tar is applied to the ears and skin ... [therapeutic effect] is thought to involve decreased epidermal proliferation ... Coal tar is classified as a human carcinogen ... Both inhalation and dermal routes of exposure are considered hazardous.
- ↑ 31.0 31.1 "COAL TAR - National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Archived from the original on 2017-05-28. Retrieved 2017-06-10.
- ↑ "IARC Monographs- Classifications". monographs.iarc.fr. Archived from the original on 2017-06-10. Retrieved 2017-06-10.
CAS No.: 8007-45-2, Agent: Coal tars (see Coal-tar distillation), Volume: 35, Sup 7, Year: 1987, Agent: Coal-tar distillation, Group: 1, Volume: 92, 100F, Year: 2012
- ↑ "COAL-TARS (Group I)" (PDF). IARC MONOGRAPHS SUPPLEMENT 7. IARC. p. 175. Archived (PDF) from the original on 2016-03-15.
Evidence for carcinogenicity to humans (sufficient)
- ↑ "Report on Carcinogens, Fourteenth Edition: Coal Tars and Coal-Tar Pitches" (PDF). National Toxicology Program, Department of Health and Human Services. Archived (PDF) from the original on 2017-02-01. Retrieved 2017-06-10.
- ↑ Quirmbach, Chuck (7 February 2017). "Milwaukee Common Council Bans Coal Tar Sealants". Wisconsin Public Radio. Archived from the original on 28 August 2019. Retrieved 28 August 2019.
- ↑ "Sun-Sensitive Drugs (Photosensitivity to Drugs)". MedicineNet. WebMD. 2008-08-22. p. 5. Archived from the original on 2013-03-17. Retrieved 8 March 2013.
- ↑ "WHO Model Prescribing Information: Drugs Used in Skin Diseases: Keratoplastic and keratolytic agents: Coal tar". apps.who.int. Archived from the original on 2017-04-20. Retrieved 2017-06-10.
keratolytic agent that inhibits excessive proliferation of epidermal cells by reducing DNA synthesis and mitotic activity to normal levels
- ↑ Heinz-Gerhard Franck (May 1963). "The Challenge in Coal Tar Chemicals". Industrial & Engineering Chemistry. 55 (5): 38–44. doi:10.1021/ie50641a006.
- ↑ Betts, WD (1997). "Tar and pitch". In John Wiley & Sons, Inc (ed.). Kirk-Othmer Encyclopedia of Chemical Technology (5th ed.). New York: John Wiley & Sons. doi:10.1002/0471238961. ISBN 9780471238966.
{{cite book}}: CS1 maint: url-status (link) - ↑ "EUR-Lex - 32013R1272 - EN - EUR-Lex". eur-lex.europa.eu. Archived from the original on 2015-10-19. Retrieved 2017-06-10.
...are classified as carcinogens of category 1B in accordance with Annex VI to Regulation (EC) No 1272/2008 of the European Parliament
- ↑ Dronsfield, Alan (1 July 2005). "Pain relief: from coal tar to paracetamol". Education in Chemistry. Vol. 42, no. 4. Royal Society of Chemistry. pp. 102–105. Archived from the original on 13 October 2017. Retrieved 14 June 2018.
- ↑ Assessment, US EPA National Center for Environmental (15 March 2009). "Hematologic Disorders". hero.epa.gov. Archived from the original on 28 July 2020. Retrieved 21 April 2020.
- ↑ "CDC – NIOSH Pocket Guide to Chemical Hazards – Coal tar pitch volatiles". www.cdc.gov. Archived from the original on 2015-12-08. Retrieved 2015-11-27.
External links
| Identifiers: |
|
|---|
| Look up coal tar in Wiktionary, the free dictionary. |
- "Coal Tar Pitch Volatiles". Occupational Safety & Health Administration. 22 March 2012. Archived from the original on 24 June 2010. Retrieved 8 March 2013.
- "NIOSH Pocket Guide to Chemical Hazards – Coal Tar Pitch Volatiles". Centers for Disease Control and Prevention. 11 April 2011. Archived from the original on 8 December 2015. Retrieved 13 September 2013.
- Erica Engelhaupt (19 November 2008). "Parking lots create sticky pollution problem". Environmental Science and Technology. 43 (1): 3. Bibcode:2009EnST...43....3E. doi:10.1021/es803118b.
- Lunge, Georg (1911). . In Chisholm, Hugh (ed.). Encyclopædia Britannica. Vol. 6 (11th ed.). Cambridge University Press. pp. 595–599.
- Pages using duplicate arguments in template calls
- CS1 errors: unsupported parameter
- CS1 maint: url-status
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles without a structure image
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Articles containing Latin-language text
- Articles with hatnote templates targeting a nonexistent page
- All articles lacking reliable references
- Articles lacking reliable references from May 2015
- Articles with invalid date parameter in template
- Chemicals that do not have a ChemSpider ID assigned
- Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference
- Antipsoriatics
- Coal
- IARC Group 1 carcinogens
- Materials
- World Health Organization essential medicines
- RTT