Coronavirus membrane protein
| Membrane protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
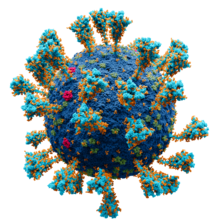 Model of the external structure of the SARS-CoV-2 virion.[1]
● Blue: envelope ● Turquoise: spike glycoprotein (S) ● Red: envelope proteins (E) ● Green: membrane proteins (M) ● Orange: glycans | |||||||||
| Identifiers | |||||||||
| Symbol | CoV_M | ||||||||
| Pfam | PF01635 | ||||||||
| InterPro | IPR002574 | ||||||||
| PROSITE | PS51927 | ||||||||
| |||||||||
The membrane (M) protein (previously called E1, sometimes also matrix protein[2]) is an integral membrane protein that is the most abundant of the four major structural proteins found in coronaviruses.[3][4][5] The M protein organizes the assembly of coronavirus virions through protein-protein interactions with other M protein molecules as well as with the other three structural proteins, the envelope (E), spike (S), and nucleocapsid (N) proteins.[4][6][7][8]
Structure
The M protein is a transmembrane protein with three transmembrane domains and is around 230 amino acid residues long.[8][9] In SARS-CoV-2, the causative agent of COVID-19, the M protein is 222 residues long.[10] Its membrane topology orients the C-terminus toward the cytosolic face of the membrane and thus into the interior of the virion. It has a short N-terminal segment and a larger C-terminal domain. Although the protein sequence is not well conserved across all coronavirus groups, there is a conserved amphipathic region near the C-terminal end of the third transmembrane segment.[8][9]
M functions as a homodimer.[4][5] Studies of the M protein in multiple coronaviruses by cryo-electron microscopy have identified two distinct functional protein conformations, thought to have different roles in forming protein-protein interactions with other structural proteins.[5] M protein of SARS-CoV-2 is homologous to the prokaryotic sugar transport protein SemiSWEET.[11]
Post-translational modifications
M is a glycoprotein whose glycosylation varies according to coronavirus subgroup; N-linked glycosylation is typically found in the alpha and gamma groups while O-linked glycosylation is typically found in the beta group.[8][9] There are some exceptions; for example, in SARS-CoV, a betacoronavirus, the M protein has one N-glycosylation site.[8][6] Glycosylation state does not appear to have a measurable effect on viral growth.[6][9][12] No other post-translational modifications have been described for the M protein.[4]
Expression and localization
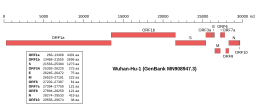 Genomic organisation of isolate Wuhan-Hu-1, the earliest sequenced sample of SARS-CoV-2, indicating the location of the M gene | |
| NCBI genome ID | 86693 |
|---|---|
| Genome size | 29,903 bases |
| Year of completion | 2020 |
| Genome browser (UCSC) | |
The gene encoding the M protein is located toward the 3' end of the virus's positive-sense RNA genome, along with the genes for the other three structural proteins and various virus-specific accessory proteins.[6][8] M is translated by membrane-bound polysomes[6] to be inserted into the endoplasmic reticulum (ER) and trafficked to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), the intracellular compartment that gives rise to the coronavirus viral envelope, or to the Golgi apparatus.[8][7][6] The exact localization is dependent on the specific virus protein.[13] Investigations of the subcellular localization of the MERS-CoV M protein found C-terminal sequence signals associated with trafficking to the Golgi.[14]
Function

The M protein is the most abundant protein in coronavirus virions.[8][5][4] It is essential for viral replication.[4]
Viral assembly
The primary function of the M protein is organizing assembly of new virions.[4] It is involved in establishing viral shape and morphology. Individual M molecules interact with each other to form the viral envelope[7][9][8] and may be able to exclude host cell proteins from the viral membrane.[5] Studies of the SARS-CoV M protein suggest that M-M interactions involve both the N- and C-termini.[6] Coronaviruses are moderately pleomorphic and conformational variations of M appear to be associated with virion size.[5]
M forms protein-protein interactions with all three other major structural proteins.[4][7] M is necessary but not sufficient for viral assembly; M and the E protein expressed together are reportedly sufficient to form virus-like particles,[7] though some reports vary depending on experimental conditions and the specific virus studied.[6][13] In some reports M appears to be capable of inducing membrane curvature,[5] though others report M alone is insufficient for this and E is required.[7] Although the E protein is not necessarily essential, it appears to be required for normal viral morphology and may be responsible for establishing curvature or initiating viral budding.[7] M also appears to have functional roles in the later stages of viral maturation, secretion, and budding.[4]
Incorporation of the spike protein (S) - which is required for assembly of infectious virions - is reported to occur though M interactions and may depend on specific conformations of M.[5][13] The conserved amphipathic region C-terminal to the third transmembrane segment is important for spike interactions.[13] Interactions with M appear to be required for correct subcellular localization of S at the viral budding site.[12] M interacts directly with the nucleocapsid (N) protein without requiring the presence of RNA.[6] This interaction appears to occur primarily through both proteins' C-termini.[4]
Interactions with the immune system

The M protein in MERS-CoV, SARS-CoV, and SARS-CoV-2 has been described as an antagonist of interferon response.[4][17]
The M protein is immunogenic and has been reported to be a determinant of humoral immunity.[4] Cytotoxic T cell responses to M have been described.[16] Antibodies to epitopes found in the M protein have been identified in patients recovered from severe acute respiratory syndrome (SARS).[18]
Other recent research has identified that SAS-COV-2 membrane protein when treated on human PBMC's causes a significant increase in pro inflammatory mediators such as TNF and IL-6.[19] The effects of exogenous SARS-COV-2 membrane protein challenge in mice was also studied. In these studies, exogenous membrane protein treated intra nasally caused a significant increase in pulmonary inflammation in mice leading to histological changes within the lungs.[20]
Host cell entry
It has been reported that human coronavirus NL63 relies on the M protein as well as the S protein to mediate host cell interactions preceding viral entry. M is thought to bind heparan sulfate proteoglycans exposed on the cell surface.[21]
Evolution and conservation
A study of SARS-CoV-2 sequences collected during the COVID-19 pandemic found that missense mutations in the M gene were relatively uncommon and suggested it was under purifying selection.[22] Similar results have been described for broader population genetics analyses over a wider range of related viruses, finding that the sequences of M and several non-structural proteins in the coronavirus genome are most subject to evolutionary constraints.[23]
References
- ^ Solodovnikov, Alexey; Arkhipova, Valeria (2021-07-29). "Достоверно красиво: как мы сделали 3D-модель SARS-CoV-2" [Truly beautiful: how we made the SARS-CoV-2 3D model] (in Russian). N+1. Archived from the original on 2021-07-30. Retrieved 30 July 2021.
- ^ Hu Y, Wen J, Tang L, Zhang H, Zhang X, Li Y, et al. (May 2003). "The M protein of SARS-CoV: basic structural and immunological properties". Genomics, Proteomics & Bioinformatics. 1 (2): 118–130. doi:10.1016/S1672-0229(03)01016-7. PMC 5172243. PMID 15626342.
- ^ Thomas S. The Structure of the Membrane Protein of SARS-CoV-2 Resembles the Sugar Transporter SemiSWEET. Pathog Immun. 2020 Oct 19;5(1):342-363.
- ^ a b c d e f g h i j k l Wong NA, Saier MH (January 2021). "The SARS-Coronavirus Infection Cycle: A Survey of Viral Membrane Proteins, Their Functional Interactions and Pathogenesis". International Journal of Molecular Sciences. 22 (3): 1308. doi:10.3390/ijms22031308. PMC 7865831. PMID 33525632.
- ^ a b c d e f g h Neuman BW, Kiss G, Kunding AH, Bhella D, Baksh MF, Connelly S, et al. (April 2011). "A structural analysis of M protein in coronavirus assembly and morphology". Journal of Structural Biology. 174 (1): 11–22. doi:10.1016/j.jsb.2010.11.021. PMC 4486061. PMID 21130884.
- ^ a b c d e f g h i Tseng YT, Wang SM, Huang KJ, Lee AI, Chiang CC, Wang CT (April 2010). "Self-assembly of severe acute respiratory syndrome coronavirus membrane protein". The Journal of Biological Chemistry. 285 (17): 12862–12872. doi:10.1074/jbc.M109.030270. PMC 2857088. PMID 20154085.
- ^ a b c d e f g Schoeman D, Fielding BC (May 2019). "Coronavirus envelope protein: current knowledge". Virology Journal. 16 (1): 69. doi:10.1186/s12985-019-1182-0. PMC 6537279. PMID 31133031.
- ^ a b c d e f g h i Masters PS (2006). "The molecular biology of coronaviruses". Advances in Virus Research. 66: 193–292. doi:10.1016/S0065-3527(06)66005-3. ISBN 9780120398690. PMC 7112330. PMID 16877062.
- ^ a b c d e J Alsaadi EA, Jones IM (April 2019). "Membrane binding proteins of coronaviruses". Future Virology. 14 (4): 275–286. doi:10.2217/fvl-2018-0144. PMC 7079996. PMID 32201500.
- ^ Cao Y, Yang R, Lee I, Zhang W, Sun J, Wang W, Meng X (June 2021). "Characterization of the SARS-CoV-2 E Protein: Sequence, Structure, Viroporin, and Inhibitors". Protein Science. 30 (6): 1114–1130. doi:10.1002/pro.4075. PMC 8138525. PMID 33813796.
- ^ Thomas S. The Structure of the Membrane Protein of SARS-CoV-2 Resembles the Sugar Transporter SemiSWEET. Pathog Immun. 2020 Oct 19;5(1):342-363
- ^ a b Voss D, Pfefferle S, Drosten C, Stevermann L, Traggiai E, Lanzavecchia A, Becker S (June 2009). "Studies on membrane topology, N-glycosylation and functionality of SARS-CoV membrane protein". Virology Journal. 6 (1): 79. doi:10.1186/1743-422X-6-79. PMC 2705359. PMID 19534833.
- ^ a b c d Ujike M, Taguchi F (April 2015). "Incorporation of spike and membrane glycoproteins into coronavirus virions". Viruses. 7 (4): 1700–1725. doi:10.3390/v7041700. PMC 4411675. PMID 25855243.
- ^ Perrier A, Bonnin A, Desmarets L, Danneels A, Goffard A, Rouillé Y, et al. (September 2019). "The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal". The Journal of Biological Chemistry. 294 (39): 14406–14421. doi:10.1074/jbc.RA119.008964. PMC 6768645. PMID 31399512.
- ^ Goodsell DS, Voigt M, Zardecki C, Burley SK (August 2020). "Integrative illustration for coronavirus outreach". PLOS Biology. 18 (8): e3000815. doi:10.1371/journal.pbio.3000815. PMC 7433897. PMID 32760062.
- ^ a b Liu J, Sun Y, Qi J, Chu F, Wu H, Gao F, et al. (October 2010). "The membrane protein of severe acute respiratory syndrome coronavirus acts as a dominant immunogen revealed by a clustering region of novel functionally and structurally defined cytotoxic T-lymphocyte epitopes". The Journal of Infectious Diseases. 202 (8): 1171–1180. doi:10.1086/656315. PMC 7537489. PMID 20831383.
- ^ Zheng Y, Zhuang MW, Han L, Zhang J, Nan ML, Zhan P, et al. (December 2020). "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling". Signal Transduction and Targeted Therapy. 5 (1): 299. doi:10.1038/s41392-020-00438-7. PMC 7768267. PMID 33372174.
- ^ Pang H, Liu Y, Han X, Xu Y, Jiang F, Wu D, et al. (October 2004). "Protective humoral responses to severe acute respiratory syndrome-associated coronavirus: implications for the design of an effective protein-based vaccine". The Journal of General Virology. 85 (Pt 10): 3109–3113. doi:10.1099/vir.0.80111-0. PMID 15448374.
- ^ Haystead, T., Lee, E., Cho, K., Gullickson, G., Hughes, P., Krafsur, G., ... & Scarneo, S. (2023). Investigation of SARS-CoV-2 individual proteins reveals the in vitro and in vivo immunogenicity of membrane protein. Scientific Reports, 13(1), 22873.
- ^ Haystead, T., Lee, E., Cho, K., Gullickson, G., Hughes, P., Krafsur, G., ... & Scarneo, S. (2023). Investigation of SARS-CoV-2 individual proteins reveals the in vitro and in vivo immunogenicity of membrane protein. Scientific Reports, 13(1), 22873.
- ^ Naskalska A, Dabrowska A, Szczepanski A, Milewska A, Jasik KP, Pyrc K (October 2019). "Membrane Protein of Human Coronavirus NL63 Is Responsible for Interaction with the Adhesion Receptor". Journal of Virology. 93 (19): e00355-19. doi:10.1128/JVI.00355-19. PMC 6744225. PMID 31315999.
- ^ Shen L, Bard JD, Triche TJ, Judkins AR, Biegel JA, Gai X (December 2021). "Emerging variants of concern in SARS-CoV-2 membrane protein: a highly conserved target with potential pathological and therapeutic implications". Emerging Microbes & Infections. 10 (1): 885–893. doi:10.1080/22221751.2021.1922097. PMC 8118436. PMID 33896413.
- ^ Cagliani R, Forni D, Clerici M, Sironi M (June 2020). "Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2". Journal of Virology. 94 (12): e00411-20. doi:10.1128/JVI.00411-20. PMC 7307108. PMID 32238584.
