Viral vector

Viral vectors (or as some types Recombinant viral vector[2]) are tools commonly used by molecular biologists to deliver genetic material into cells. This process can be performed inside a living organism (in vivo) or in cell culture (in vitro). Viruses have evolved specialized molecular mechanisms to efficiently transport their genomes inside the cells they infect. Delivery of genes or other genetic material by a vector is termed transduction and the infected cells are described as transduced. Molecular biologists first harnessed this machinery in the 1970s. Paul Berg used a modified SV40 virus containing DNA from the bacteriophage λ to infect monkey kidney cell maintained in culture.[3]
In addition to their use in molecular biology research, viral vectors are used for gene therapy and the development of vaccines. Vectors can either integrate into a cell's genome or transiently express a gene with non-integrative vectors.[4]: 50
Properties of viral vector
Viral Vectors are tailored to their specific applications but generally share a few key properties.
- Safety: Although viral vectors are occasionally created from pathogenic viruses, they are modified in such a way as to minimize the risk of handling them. This usually involves the deletion of a part of the viral genome critical for viral replication. Such a virus can efficiently infect cells but, once the infection has taken place, requires a helper virus to provide the missing proteins for production of new virions.[6][7][8]
- Low toxicity: The viral vector should have a minimal effect on the physiology of the cell it infects.[8]
- Stability: Some viruses are genetically unstable and can rapidly rearrange their genome and hence should be avoided in their design.[8]
- Cell type specificity: Viral vector can be modified to target the virus to a specific kind of cell. Viruses modified in this manner are said to be pseudotyped.[8][9]
- Identification: Viral vectors are often given certain genes that help identify which cells took up the viral genes, these genes are called markers. [8]
- A common marker is resistance to a certain antibiotic, the cells can then be isolated easily, as those that have not taken up the viral vector genes do not have antibiotic resistance[8]
Applications

Basic research
Viral vectors were originally developed as an alternative to transfection of naked DNA for molecular genetics experiments. Compared to traditional methods of transfection, transduction can ensure that nearly 100% of cells are infected without severely affecting cell viability. Furthermore, some viruses integrate into the cell genome facilitating stable expression.[7][11][12]
Protein coding genes can be expressed using viral vectors, commonly to study the function of the particular protein. Viral vectors, stably expressing marker genes such as GFP are widely used to permanently label cells to track them and their progeny.[13][14]
Gene insertion, can be done with viral vectors, and has certain advantages over gene knockout. [15][16]
Gene therapy
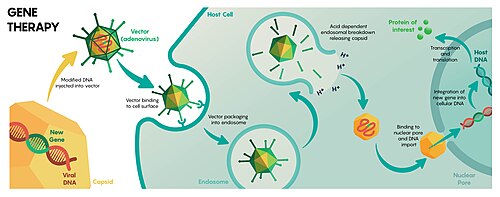
Gene therapy is a technique for correcting defective genes responsible for disease development. In the future, gene therapy may provide a way to cure genetic disorders, such as severe combined immunodeficiency, cystic fibrosis or even haemophilia A. Because these diseases result from mutations in the DNA sequence for specific genes, gene therapy trials have used viruses to deliver unmutated copies of these genes to the cells of the patient's body. There have been a huge number of laboratory successes with gene therapy. However, several problems of viral gene therapy must be overcome before it gains widespread use. Immune response to viruses not only impedes the delivery of genes to target cells but can cause severe complications for the patient. In one of the early gene therapy trials in 1999 this led to the death of Jesse Gelsinger, who was treated using an adenoviral vector.[17][18][11][19]
Some viral vectors, for instance gamma-retroviruses, insert their genomes at a seemingly random location on one of the host chromosomes, which can disturb the function of cellular genes and lead to cancer. In a severe combined immunodeficiency retroviral gene therapy trial conducted in 2002, four of the patients developed leukemia as a consequence of the treatment;[20] three of the patients recovered after chemotherapy.[21] Adeno-associated virus-based vectors are much safer in this respect as they always integrate at the same site in the human genome, with applications in various disorders, such as Alzheimer's disease.[22]
Vaccines
A live vector vaccine is a vaccine that uses an organism (typically virus or bacterium) that does not cause disease to transport the pathogen genes into the body in order to stimulate an immune response.[23]
Viruses expressing pathogen proteins are currently being developed as vaccines against these pathogens, based on the same rationale as DNA vaccines. The genes used in such vaccines are usually antigen coding surface proteins from the pathogenic organism. They are then inserted into the genome of a non-pathogenic organism, where they are expressed on the organism's surface and can elicit an immune response. Insert protein-coding gene from pathogenic virus into the genome of a non-pathogenic virus, then use the non-pathogenic viruses as vector vaccine to infect the host cells which express pathogenic surface protein and cause immune responses. [24][25][26][27]
Unlike attenuated vaccines, viral vector vaccines lack other pathogen genes required for replication, so infection by the pathogen is impossible. Adenoviruses are being actively developed as vaccine vectors.[28][29]
-
Adenoviral vector-based vaccine strategy for creating an effective protective immunity[30]
-
Mechanism of viral vector vaccines[31]
Medicine delivery
A strain of canarypox virus modified to carry feline interleukin-2 is used to treat cats with fibrosarcoma.[32]
Challenges in application
The choice of a viral vector to deliver genetic material to cells comes with some logistical problems. There are a limited number of viral vectors available for therapeutic use. Any of these few viral vectors can cause the body to develop an immune response if the vector is seen as a foreign invader. Once used, the viral vector cannot be effectively used in the patient again because it will be recognized by the body. If the vaccine or gene therapy fails in clinical trials, the virus can't be used again in the patient for a different vaccine or gene therapy .[33][34][35]
Pre-existing immunity against the viral vector could also be present in the patient, rendering the therapy ineffective for that patient.[34][36] Because priming with a naked DNA vaccine and boosting with a viral vector results in a robust immune response via yet indefinite mechanism(s), despite pre-existing viral vector immunity, this vaccination strategy can counteract this problem.[37] However, this method may present another expense and obstacle in the vaccine distribution process. Pre-existing immunity may also be challenged by increasing vaccine dose or changing the vaccination route.[38] Some shortcomings of viral vectors (such as genotoxicity and low transgenic expression) can be overcome through the use of hybrid vectors.[39]
Types
Retroviruses
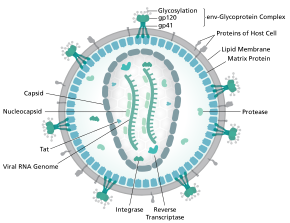
Retroviruses are one of the mainstays of current gene therapy approaches. The recombinant retroviruses such as the Moloney murine leukemia virus have the ability to integrate into the host genome in a stable fashion. They contain a reverse transcriptase to make a DNA copy of the RNA genome, and an integrase that allows integration into the host genome. They have been used in a number of FDA-approved clinical trials such as the SCID-X1 trial.[40]
Retroviral vectors can either be replication-competent or replication-defective. Replication-defective vectors are the most common choice in studies because the viruses have had the coding regions for the genes necessary for additional rounds of virion replication and packaging replaced with other genes, or deleted. These virus are capable of infecting their target cells and delivering their viral payload, but then fail to continue the typical lytic pathway that leads to cell lysis and death.[41][42][43][44]
Conversely, replication-competent viral vectors contain all necessary genes for virion synthesis, and continue to propagate themselves once infection occurs. Because the viral genome for these vectors is much lengthier, the length of the actual inserted gene of interest is limited compared to the possible length of the insert for replication-defective vectors. While this limits the introduction of many genomic sequences, most cDNA sequences can still be accommodated.The primary drawback to use of retroviruses such as the Moloney retrovirus involves the requirement for cells to be actively dividing for transduction.[45][46][47][42]
There is concern that insertional mutagenesis due to integration into the host genome might lead to cancer or leukemia. This concern remained theoretical until gene therapy for ten SCID-X1 patients using Moloney murine leukemia virus[48] resulted in two cases of leukemia caused by activation of the LMO2 oncogene due to nearby integration of the vector.[49]
Lentiviruses

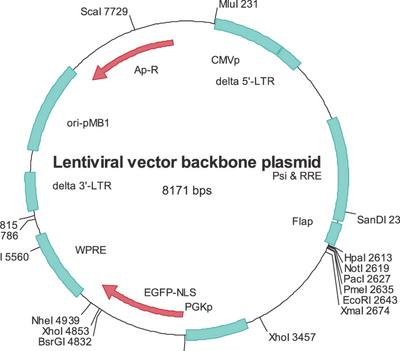
Lentiviruses are a subclass of retroviruses. They are sometimes used as vectors for gene therapy thanks to their ability to integrate into the genome of non-dividing cells, which is a unique feature of lentiviruses, as other retroviruses can infect only dividing cells. The viral genome in the form of RNA is reverse-transcribed when the virus enters the cell to produce DNA, which is then inserted into the genome at a random position (although recent findings suggest that the insertion of viral DNA is not random but directed to specific active genes and related to genome organisation[50]) by the viral integrase enzyme. The vector, now called a provirus, remains in the genome and is passed on to the progeny of the cell when it divides. There are, as yet, no techniques for determining the site of integration, which can pose a problem. The provirus can disturb the function of cellular genes and lead to activation of oncogenes promoting the development of cancer, which raises concerns for possible applications of lentiviruses in gene therapy. However, studies have shown that lentivirus vectors have a lower tendency to integrate in places that potentially cause cancer than gamma-retroviral vectors.[51] More specifically, one study found that lentiviral vectors did not cause either an increase in tumor incidence or an earlier onset of tumors in a mouse strain prone to tumors.[52] Moreover, clinical trials that utilized lentiviral vectors to deliver gene therapy for the treatment of HIV experienced no increase in mutagenic or oncologic events.[53] Versions of the lentiviral vector have been developed that do not integrate their genetic material into the cell's genome but only transiently express genes.[4]: 50
For safety reasons, lentiviral vectors never carry the genes required for their replication. To produce a lentivirus, several plasmids are transfected into a so-called packaging cell line, commonly HEK 293. One or more plasmids, generally referred to as packaging plasmids, encode the virion proteins, such as the capsid and the reverse transcriptase. Another plasmid contains the genetic material to be delivered by the vector. It is transcribed to produce the single-stranded RNA viral genome and is marked by the presence of the ψ (psi) sequence. This sequence is used to package the genome into the virion.[54][55][56]
Adenoviruses

As opposed to lentiviruses, adenoviral DNA does not integrate into the genome and is not replicated during cell division.[58]: 5 This limits their use in basic research, although adenoviral vectors are still used in in vitro and also in vivo experiments.[59] Their primary applications are in gene therapy and vaccination. Since humans commonly come in contact with adenoviruses, which cause respiratory, gastrointestinal and eye infections, majority of patients have already developed neutralizing antibodies which can inactivate the virus before it can reach the target cell. To overcome this problem scientists are currently investigating adenoviruses that infect different species to which humans do not have immunity, for example, the chimpanzee adenovirus used as a vector to transport SARS-CoV-2 spike gene in Oxford AstraZeneca COVID vaccine. PEGylation of adenoviruses for gene therapy can help prevent adverse reactions due to pre-existing adenovirus immunity.[60]
Adeno-associated viruses
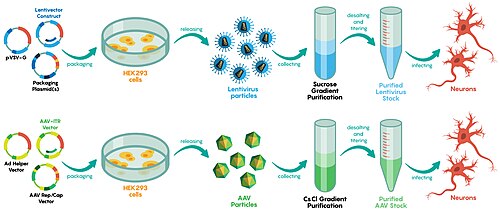
Adeno-associated virus (AAV) is a small virus that infects humans and some other primate species. AAV is not currently known to cause disease, and causes a very mild immune response. AAV can infect both dividing and non-dividing cells and may incorporate its genome into that of the host cell. Moreover, AAV mostly stays as episomal (replicating without incorporation into the chromosome); performing long and stable expression.[61] These features make AAV a very attractive candidate for creating viral vectors for gene therapy.[3] However, AAV can only bring up to 5kb which is considerably small compared to AAV's original capacity.[61]
Adeno-associated viral vectors have been engineered to evade virus recognition by TLR9 receptors by incorporating TLR9-inhibiting genes into the vector.[62]
Furthermore, because of its potential use as a gene therapy vector, researchers have created an altered AAV called self-complementary adeno-associated virus (scAAV). Whereas AAV packages a single strand of DNA and requires the process of second-strand synthesis, scAAV packages both strands which anneal together to form double stranded DNA. By skipping second strand synthesis scAAV allows for rapid expression in the cell.[63]
Plant viruses

Plant viruses can be used to engineer viral vectors, tools commonly used to deliver genetic material into plant cells; they are also sources of biomaterials and nanotechnology devices.[64][65] Tobacco mosaic virus (TMV) is the first virus to be discovered. Viral vectors based on tobacco mosaic virus include those of the magnICON[66] and TRBO plant expression technologies.[64]
Hybrids
Hybrid vectors are vector viruses that are genetically engineered to have qualities of more than one vector. Viruses are altered to avoid the shortcomings of typical viral vectors, which may have limited loading capacity, immunogenicity, genotoxicity, and fail to support long-term adequate transgenic expression. Through the replacement of undesirable elements with desired abilities, hybrid vectors may in the future outperform standard transfection vectors in terms of safety and therapeutic efficiency.[39]
See also
References
- ↑ Capasso, Cristian; Hirvinen, Mari; Cerullo, Vincenzo (December 2013). "Beyond Gene Delivery: Strategies to Engineer the Surfaces of Viral Vectors". Biomedicines. 1 (1): 3–16. doi:10.3390/biomedicines1010003. ISSN 2227-9059. PMC 5423465. PMID 28548054.
- ↑ Chen, Shih-Heng; Haam, Juhee; Walker, Mitzie; Scappini, Erica; Naughton, John; Martin, Negin P. (April 2019). "Recombinant Viral Vectors as Neuroscience Tools". Current Protocols in Neuroscience. 87 (1): e67. doi:10.1002/cpns.67. ISSN 1934-8576. Archived from the original on 2022-06-07. Retrieved 2024-01-11.
- ↑ 3.0 3.1 Goff SP, Berg P (December 1976). "Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cell". Cell. 9 (4 PT 2): 695–705. doi:10.1016/0092-8674(76)90133-1. PMID 189942. S2CID 41788896.
- ↑ 4.0 4.1 Nóbrega, Clévio (2020). A handbook of gene and cell therapy. Liliana Mendonça, Carlos A. Matos. Cham: Springer. ISBN 978-3-030-41333-0. OCLC 1163431307. Archived from the original on 2023-03-04. Retrieved 2023-12-31.
- ↑ Tolmachov, Oleg; Tolmachova, Tanya; Al-Allaf, Faisal A. (20 July 2011). "Designing Lentiviral Gene Vectors". Viral Gene Therapy. IntechOpen.
- ↑ "6 Questions (and Answers) About Viral Vector Vaccines" (PDF). NIH. Archived (PDF) from the original on 24 January 2024. Retrieved 24 January 2024.
- ↑ 7.0 7.1 Warnock, James N.; Daigre, Claire; Al-Rubeai, Mohamed (2011). "Introduction to Viral Vectors". Viral Vectors for Gene Therapy. 737: 1–25. doi:10.1007/978-1-61779-095-9_1. ISSN 1940-6029. Archived from the original on 2023-10-03. Retrieved 2024-01-05.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 "Viral Vectors - US". www.thermofisher.com. Archived from the original on 1 June 2023. Retrieved 24 January 2024.
- ↑ Rittiner, Joseph Edward; Moncalvo, Malik; Chiba-Falek, Ornit; Kantor, Boris (2020). "Gene-Editing Technologies Paired With Viral Vectors for Translational Research Into Neurodegenerative Diseases". Frontiers in Molecular Neuroscience. 13. doi:10.3389/fnmol.2020.00148. ISSN 1662-5099. PMID 32903507.
- ↑ Kumar, Priti; Nagarajan, Arvindhan; Uchil, Pradeep D. (1 July 2019). "Electroporation". Cold Spring Harbor Protocols. 2019 (7). doi:10.1101/pdb.top096271. ISSN 1559-6095.
- ↑ 11.0 11.1 Ghosh, Sumit; Brown, Alex M.; Jenkins, Chris; Campbell, Katie (1 March 2020). "Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges". Applied Biosafety: Journal of the American Biological Safety Association. 25 (1): 7–18. doi:10.1177/1535676019899502. ISSN 1535-6760. PMC 9134621. PMID 36033383.
- ↑ Tomanin, R.; Scarpa, M. (December 2004). "Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction". Current Gene Therapy. 4 (4): 357–372. doi:10.2174/1566523043346011. ISSN 1566-5232. Archived from the original on 2023-11-18. Retrieved 2024-01-16.
- ↑ Piovesan, Allison; Antonaros, Francesca; Vitale, Lorenza; Strippoli, Pierluigi; Pelleri, Maria Chiara; Caracausi, Maria (4 June 2019). "Human protein-coding genes and gene feature statistics in 2019". BMC research notes. 12 (1): 315. doi:10.1186/s13104-019-4343-8. ISSN 1756-0500. Archived from the original on 9 January 2024. Retrieved 9 January 2024.
- ↑ Suzuki, Yasutsugu; Suzuki, Youichi (20 July 2011). "Gene Regulatable Lentiviral Vector System". Viral Gene Therapy. IntechOpen. Archived from the original on 30 December 2023. Retrieved 15 January 2024.
- ↑ Papapetrou, Eirini P; Schambach, Axel (April 2016). "Gene Insertion Into Genomic Safe Harbors for Human Gene Therapy". Molecular Therapy. 24 (4): 678–684. doi:10.1038/mt.2016.38. ISSN 1525-0016. Archived from the original on 2022-10-02. Retrieved 2024-01-08.
- ↑ Hendrie, Paul C.; Russell, David W. (July 2005). "Gene Targeting with Viral Vectors". Molecular Therapy. 12 (1): 9–17. doi:10.1016/j.ymthe.2005.04.006. Archived from the original on 2024-01-20. Retrieved 2024-01-20.
- ↑ Beardsley T (February 2000). "A tragic death clouds the future of an innovative treatment method". Scientific American.
- ↑ Lundstrom, Kenneth (21 May 2018). "Viral Vectors in Gene Therapy". Diseases. 6 (2): 42. doi:10.3390/diseases6020042. ISSN 2079-9721. PMC 6023384. PMID 29883422.
- ↑ Zhao, Zongmin; Anselmo, Aaron C.; Mitragotri, Samir (January 2022). "Viral vector‐based gene therapies in the clinic". Bioengineering & Translational Medicine. 7 (1). doi:10.1002/btm2.10258. ISSN 2380-6761. Archived from the original on 2024-01-16. Retrieved 2024-01-16.
- ↑ McDowell N (15 January 2003). "New cancer case halts US gene therapy trials". New Scientist. Archived from the original on 22 October 2008. Retrieved 31 December 2023.
- ↑ Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, et al. (July 2010). "Efficacy of gene therapy for X-linked severe combined immunodeficiency". The New England Journal of Medicine. 363 (4): 355–64. doi:10.1056/NEJMoa1000164. PMC 2957288. PMID 20660403.
- ↑ Sasmita AO (April 2019). "Current viral-mediated gene transfer research for treatment of Alzheimer's disease". Biotechnology & Genetic Engineering Reviews. 35 (1): 26–45. doi:10.1080/02648725.2018.1523521. PMID 30317930. S2CID 52978228.
- ↑ "Live-Vector Vaccine". American Institute of Chemical Engineers. 17 December 2014. Archived from the original on 2021-02-03. Retrieved 2021-02-03.
- ↑ McCann, Naina; O'Connor, Daniel; Lambe, Teresa; Pollard, Andrew J. (August 2022). "Viral vector vaccines". Current Opinion in Immunology. 77: 102210. doi:10.1016/j.coi.2022.102210. ISSN 1879-0372. PMID 35643023.
- ↑ Travieso, Tatianna; Li, Jenny; Mahesh, Sneha; Mello, Juliana Da Fonzeca Redenze E.; Blasi, Maria (4 July 2022). "The use of viral vectors in vaccine development". npj Vaccines. 7 (1): 75. doi:10.1038/s41541-022-00503-y. ISSN 2059-0105. PMC 9253346. PMID 35787629.
- ↑ Ura, Takehiro; Okuda, Kenji; Shimada, Masaru (September 2014). "Developments in Viral Vector-Based Vaccines". Vaccines. 2 (3): 624–641. doi:10.3390/vaccines2030624. ISSN 2076-393X. Archived from the original on 2022-09-08. Retrieved 2024-01-10.
- ↑ "What are viral vector-based vaccines and how could they be used against COVID-19? | Gavi, the Vaccine Alliance". www.gavi.org. Archived from the original on 11 November 2021. Retrieved 12 January 2024.
- ↑ Badgett, Marty R.; Auer, Alexandra; Carmichael, Leland E.; Parrish, Colin R.; Bull, James J. (October 2002). "Evolutionary Dynamics of Viral Attenuation". Journal of Virology. 76 (20): 10524–10529. doi:10.1128/JVI.76.20.10524-10529.2002. ISSN 0022-538X. Archived from the original on 2023-11-17. Retrieved 2024-01-09.
- ↑ Chang, Jun (15 February 2021). "Adenovirus Vectors: Excellent Tools for Vaccine Development". Immune Network. 21 (1): e6. doi:10.4110/in.2021.21.e6. ISSN 1598-2629. Archived from the original on 20 October 2022. Retrieved 22 January 2024.
- ↑ Elkashif, Ahmed; Alhashimi, Marwa; Sayedahmed, Ekramy E; Sambhara, Suryaprakash; Mittal, Suresh K (January 2021). "Adenoviral vector‐based platforms for developing effective vaccines to combat respiratory viral infections". Clinical & Translational Immunology. 10 (10). doi:10.1002/cti2.1345. ISSN 2050-0068.
- ↑ Deng, Shaofeng; Liang, Hui; Chen, Pin; Li, Yuwan; Li, Zhaoyao; Fan, Shuangqi; Wu, Keke; Li, Xiaowen; Chen, Wenxian; Qin, Yuwei; Yi, Lin; Chen, Jinding (18 July 2022). "Viral Vector Vaccine Development and Application during the COVID-19 Pandemic". Microorganisms. 10 (7): 1450. doi:10.3390/microorganisms10071450. ISSN 2076-2607. PMC 9317404. PMID 35889169.
- ↑ "EPAR summary for the public: Oncept IL-2 (Feline interleukin-2 recombinant canary pox virus) [EMA/151380/2013 EMEA/V/C/002562]" (PDF). European Medical Agency. 2013. Archived (PDF) from the original on 2023-03-22. Retrieved 2023-12-31.
- ↑ van der Loo, Johannes C.M.; Wright, J. Fraser (15 April 2016). "Progress and challenges in viral vector manufacturing". Human Molecular Genetics. 25 (R1): R42–R52. doi:10.1093/hmg/ddv451. PMC 4802372. PMID 26519140. Archived from the original on 7 January 2024. Retrieved 7 January 2024.
- ↑ 34.0 34.1 Nayak S, Herzog RW (March 2010). "Progress and prospects: immune responses to viral vectors". Gene Therapy. 17 (3): 295–304. doi:10.1038/gt.2009.148. PMC 3044498. PMID 19907498.
- ↑ Zhou HS, Liu DP, Liang CC (November 2004). "Challenges and strategies: the immune responses in gene therapy". Medicinal Research Reviews. 24 (6): 748–61. doi:10.1002/med.20009. PMID 15250039. S2CID 17622444.
- ↑ Crommelin DJ, Sindelar RD, Meibohm B (2008). Pharmaceutical Biotechnology: Fundamentals and application. London: Taylor & Francis. ISBN 978-1420044379.
- ↑ Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ (January 2003). "Overcoming immunity to a viral vaccine by DNA priming before vector boosting". Journal of Virology. 77 (1): 799–803. doi:10.1128/JVI.77.1.799-803.2003. PMC 140625. PMID 12477888.
- ↑ Pandey A, Singh N, Vemula SV, Couëtil L, Katz JM, Donis R, et al. (2012). Subbiah E (ed.). "Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine". PLOS ONE. 7 (3): e33428. Bibcode:2012PLoSO...733428P. doi:10.1371/journal.pone.0033428. PMC 3303828. PMID 22432020.
- ↑ 39.0 39.1 Huang, Shuohao; Kamihira, Masamichi (2013). "Development of hybrid viral vectors for gene therapy". Biotechnology Advances. 31 (2): 208–223. doi:10.1016/j.biotechadv.2012.10.001. ISSN 1873-1899. Archived from the original on 2023-02-18. Retrieved 2024-01-14.
- ↑ Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. (April 2000). "Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease". Science. 288 (5466): 669–72. Bibcode:2000Sci...288..669C. doi:10.1126/science.288.5466.669. PMID 10784449.
- ↑ Kurian, K. M.; Watson, C. J.; Wyllie, A. H. (1 August 2000). "Retroviral vectors". Molecular Pathology. 53 (4): 173–176. doi:10.1136/mp.53.4.173. ISSN 1366-8714. Archived from the original on 21 March 2023. Retrieved 8 January 2024.
- ↑ 42.0 42.1 Cornetta, Kenneth; Lin, Tsai-Yu; Pellin, Danilo; Kohn, Donald B. (9 March 2023). "Meeting FDA Guidance recommendations for replication-competent virus and insertional oncogenesis testing". Molecular Therapy. Methods & Clinical Development. 28: 28–39. doi:10.1016/j.omtm.2022.11.009. ISSN 2329-0501. Archived from the original on 11 August 2023. Retrieved 23 January 2024.
- ↑ Dudek, Tim; Knipe, David M. (5 January 2006). "Replication-defective viruses as vaccines and vaccine vectors". Virology. 344 (1): 230–239. doi:10.1016/j.virol.2005.09.020. ISSN 0042-6822. Archived from the original on 26 May 2023. Retrieved 22 January 2024.
- ↑ Matuskova, Miroslava; Durinikova, Erika (16 March 2016). "Retroviral Vectors in Gene Therapy". Advances in Molecular Retrovirology. IntechOpen. ISBN 978-953-51-2261-6. Archived from the original on 18 November 2023. Retrieved 22 January 2024.
- ↑ Ayuk, F.A.; Zander, A.R.; Fehse, B. (1 November 2001). "T Lymphocytes as Targets of Gene Transfer with Moloney-Type Retroviral Vectors". Current Gene Therapy. 1 (4): 325–337. doi:10.2174/1566523013348274. Archived from the original on 16 January 2024. Retrieved 15 January 2024.
- ↑ Varmus, Harold; Coffin, John M.; Hughes, Stephen H., eds. (1997). "Principles of Retroviral Vector Design". Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-571-2. PMID 21433340. Archived from the original on 2019-10-09. Retrieved 2023-12-31.
- ↑ Coffin, John M.; Hughes, Stephen H.; Varmus, Harold E. (1997). "Principles of Retroviral Vector Design". Retroviruses. Cold Spring Harbor Laboratory Press. Archived from the original on 2023-11-25. Retrieved 2024-01-13.
- ↑ Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. (April 2002). "Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy". The New England Journal of Medicine. 346 (16): 1185–93. doi:10.1056/NEJMoa012616. PMID 11961146.
- ↑ Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. (October 2003). "LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1". Science. 302 (5644): 415–9. Bibcode:2003Sci...302..415H. doi:10.1126/science.1088547. PMID 14564000. S2CID 9100335.
- ↑ Marini B, Kertesz-Farkas A, Ali H, Lucic B, Lisek K, Manganaro L, et al. (May 2015). "Nuclear architecture dictates HIV-1 integration site selection" (PDF). Nature. 521 (7551): 227–31. Bibcode:2015Natur.521..227M. doi:10.1038/nature14226. hdl:11368/2844160. PMID 25731161. S2CID 974969. Archived (PDF) from the original on 2022-08-13. Retrieved 2023-12-31.
- ↑ Cattoglio C, Facchini G, Sartori D, Antonelli A, Miccio A, Cassani B, et al. (September 2007). "Hot spots of retroviral integration in human CD34+ hematopoietic cells". Blood. 110 (6): 1770–8. doi:10.1182/blood-2007-01-068759. PMID 17507662. S2CID 8715798.
- ↑ Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. (June 2006). "Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration". Nature Biotechnology. 24 (6): 687–96. doi:10.1038/nbt1216. PMID 16732270. S2CID 8966580.
- ↑ Lidonnici MR, Paleari Y, Tiboni F, Mandelli G, Rossi C, Vezzoli M, et al. (December 2018). "Multiple Integrated Non-clinical Studies Predict the Safety of Lentivirus-Mediated Gene Therapy for β-Thalassemia". Molecular Therapy: Methods & Clinical Development. 11: 9–28. doi:10.1016/j.omtm.2018.09.001. PMC 6178212. PMID 30320151.
- ↑ Stepanenko, A. A.; Dmitrenko, V. V. (15 September 2015). "HEK293 in cell biology and cancer research: phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution". Gene. 569 (2): 182–190. doi:10.1016/j.gene.2015.05.065. ISSN 1879-0038. Archived from the original on 16 January 2024. Retrieved 13 January 2024.
- ↑ Tan, Evan; Chin, Cara Sze Hui; Lim, Zhi Feng Sherman; Ng, Say Kong (13 December 2021). "HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors". Frontiers in Bioengineering and Biotechnology. 9: 796991. doi:10.3389/fbioe.2021.796991. ISSN 2296-4185. Archived from the original on 18 April 2023. Retrieved 18 January 2024.
- ↑ "Biosafety Considerations for Research with Lentiviral Vectors" (PDF). NIH.gov. NIH. Archived (PDF) from the original on 1 August 2023. Retrieved 23 January 2024.
- ↑ Padilla-Sanchez V (2021-07-24), Adenovirus D26 Structural Model at Atomic Resolution, doi:10.5281/zenodo.5132873, retrieved 2021-07-24
- ↑ Bulcha JT, Wang Y, Ma H, Tai PW, Gao G (February 2021). "Viral vector platforms within the gene therapy landscape". Signal Transduction and Targeted Therapy. 6 (1): 53. doi:10.1038/s41392-021-00487-6. PMC 7868676. PMID 33558455.
- ↑ Ramos-Kuri M, Rapti K, Mehel H, Zhang S, Dhandapany PS, Liang L, et al. (November 2015). "Dominant negative Ras attenuates pathological ventricular remodeling in pressure overload cardiac hypertrophy". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1853 (11 Pt A): 2870–84. doi:10.1016/j.bbamcr.2015.08.006. PMC 4715892. PMID 26260012.
- ↑ Seregin SS, Amalfitano A (2009). "Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors". Expert Opinion on Biological Therapy. 9 (12): 1521–1531. doi:10.1517/14712590903307388. PMID 19780714. S2CID 21927486.
- ↑ 61.0 61.1 Nussbaum, Robert L; McInnes, Roderick R; Willard, Huntington F (2015). Thompson & Thompson Genetics in Medicine. Canada: ELSEVIER. p. 278. ISBN 978-1-4377-0696-3.
- ↑ Chan YK, Wang SK, Church GM (2021). "Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses". Science Translational Medicine. 13 (588): eabd3438. doi:10.1126/scitranslmed.abd3438. PMC 8409505. PMID 33568518.
- ↑ McCarty DM, Monahan PE, Samulski RJ (August 2001). "Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis". Gene Therapy. 8 (16): 1248–54. doi:10.1038/sj.gt.3301514. PMID 11509958.
- ↑ 64.0 64.1 Abrahamian, Peter; Hammond, Rosemarie W.; Hammond, John (2020-06-10). "Plant Virus-Derived Vectors: Applications in Agricultural and Medical Biotechnology". Annual Review of Virology. 7 (1): 513–535. doi:10.1146/annurev-virology-010720-054958. ISSN 2327-0578. PMID 32520661. S2CID 219588089.
- ↑ Pasin, Fabio; Menzel, Wulf; Daròs, José-Antonio (June 2019). "Harnessed viruses in the age of metagenomics and synthetic biology: an update on infectious clone assembly and biotechnologies of plant viruses". Plant Biotechnology Journal. 17 (6): 1010–1026. doi:10.1111/pbi.13084. ISSN 1467-7652. PMC 6523588. PMID 30677208.
- ↑ "magnICON". Archived from the original on 2021-01-10. Retrieved 2023-12-31.
Further reading
- Torashima, T.; Koyama, C.; Higashida, H.; Hirai, H. (2007). "Production of neuron-preferential lentiviral vectors". Protocol Exchange. doi:10.1038/nprot.2007.89.
- Okada, Y.; Ikawa, M. (2007). "Placenta specific gene manipulation by transducing zona-free blastocyst using lentiviral vector". Protocol Exchange. doi:10.1038/nprot.2007.62.
- Fry JW, Wood KJ (8 June 1999). "A comparison of vectors in use for clinical gene transfer". Expert Reviews in Molecular Medicine. Archived from the original on 24 May 2011. Retrieved 31 December 2023.
![Adenoviral vector-based vaccine strategy for creating an effective protective immunity[30]](https://nccommons.org/media/3/3c/Cti21345-fig-0005-m.png)
![Mechanism of viral vector vaccines[31]](https://upload.wikimedia.org/wikipedia/commons/thumb/0/0b/Microorganisms-10-01450-g002.jpg/490px-Microorganisms-10-01450-g002.jpg)