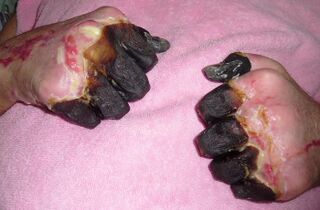
Septicemic plague
| Septicemia plague | |
|---|---|
| |
| Specialty | Infectious disease |
| Symptoms | Fever, chills, low blood pressure, organ failure, shock |
| Causes | Yersinia pestis |
| Diagnostic method | Blood sample, culture sample |
| Prevention | Insecticide, avoid contact with dead rodents |
| Treatment | Streptomycin, gentamicin, tetracycline |
Septicemic plague is one of the three forms of plague, and is caused by Yersinia pestis, a gram-negative species of bacterium. It is a systemic disease involving infection of the blood, and is most commonly spread by bites from infected fleas .[1]
Complications can include disseminated intravascular coagulation, and is always fatal when untreated. The other varieties of the plague are bubonic plague and pneumonic plague.[1]
Signs and symptoms
- Abdominal pain
- Bleeding under skin due to blood clotting problems
- Bleeding from mouth, nose or rectum
- Gastrointestinal symptoms, including nausea, vomiting, which can be with blood, and diarrhea
- Fever
- Chills
- Low blood pressure
- Organ failure
- Shock
- Gangrene in extremities, mostly fingers, nose, and toes
- Difficulty breathing
These symptoms are common to many human illnesses, and are not considered, in and of themselves, to signify infection with any form of plague.It is important to note that septicemic plague may be asymptomatic[3]
Cause

Human Yersinia infections most commonly result from the bite of an infected flea or occasionally an infected mammal, but like most bacterial systemic diseases, the disease may be transmitted through an opening in the skin or by inhaling infectious droplets of moisture from sneezes or coughs. In both cases septicemic plague need not be the result, and in particular, not the initial result, but it occasionally happens that bubonic plague for example leads to infection of the blood, and septicemic plague results. If the bacteria happen to enter the bloodstream rather than the lymph or lungs, they multiply in the blood, causing sepsis. In septicemic plague, bacterial endotoxins cause disseminated intravascular coagulation (DIC), where tiny blood clots form throughout the body, commonly resulting in localised ischemic necrosis, tissue death from lack of circulation and perfusion.[4][5][6]
DIC results in depletion of the body's clotting resources, so that it can no longer control bleeding. Consequently, the unclotted blood bleeds into the skin and other organs, leading to red or black patchy rash and to hematemesis or hemoptysis . The rash may cause bumps on the skin that look somewhat like insect bites, usually red, sometimes white in the center.[7][8]
Septicemic plague is caused by horizontal and direct transmission. Horizontal transmission is the transmitting of a disease from one individual to another regardless of blood relation. Direct transmission occurs from close physical contact with individuals, through common air usage, from direct bite from a flea or an infected rodent. [9][10][11] Significant carriers of the bacteria in the United States include:[12]
The bacteria are cosmopolitan, mainly in rodents in all continents except Australia and Antarctica. The greatest frequency of human plague infections occurs in Africa. The bacteria most commonly appear in rural areas and wherever there is poor sanitation, overcrowding, and high rodent populations in urban areas. Outdoor activities such as hiking, camping, or hunting where plague-infected animals may be found, increase the risk of contracting septicemic plague, and so do certain occupations such as veterinary or other animal-related work.[1]
Mechanism
Pathogenesis due to Y. pestis infection of mammalian hosts is due to several factors, including an ability of these bacteria to suppress and avoid normal immune system responses such as phagocytosis and antibody production. Flea bites allow for the bacteria to pass the skin barrier. Y. pestis expresses a plasmin activator that is an important virulence factor for pneumonic plague and that might degrade on blood clots to facilitate systematic invasion.[13] Many of the bacteria's virulence factors are antiphagocytic in nature. Two important antiphagocytic antigens, named F1 (fraction 1) and V or LcrV, are both important for virulence.[14] These antigens are produced by the bacterium at normal human body temperature. Furthermore, Y. pestis survives and produces F1 and V antigens while it is residing within white blood cells such as monocytes, but not in neutrophils. Natural or induced immunity is achieved by the production of specific opsonic antibodies against F1 and V antigens; antibodies against F1 and V induce phagocytosis by neutrophils.[15]
Diagnosis

In terms of the diagnosis for Septicemic plague we find that a doctor or veterinarian will perform a physical exam which includes asking about the medical history and possible sources of exposure.[16] The following possible test could include:
- Blood samples (detecting antibodies)[17]
- Culture samples of body fluids[18]
- Liver testing[19]
- Checking lymphatic system for signs of infection[20]
- Checking for lung infection[21]
Differential diagnosis
The DDx for Septicemic plague is as follows:[5]
Prevention
The following precautions may be be used to avoid infection:[1][21]
- Caregivers of infected patients should wear masks, gloves, goggles and gowns
- Take antibiotics if close contact with infected patient has occurred
- Use insecticides throughout house
- Avoid contact with dead rodents or sick cats
- Set traps if mice or rats are present around the house
- Do not allow family pets to roam in areas where plague is common
- Flea control and treatment for rodents
Treatment

Starting antibiotics early is a first step in treating septicemic plague in humans. One of the following antibiotics may be used:[5][18][22]
Lymph nodes may require draining and the patient will need close monitoring.[1]
In animals, antibiotics such as tetracycline or doxycycline can be used. Intravenous drip may be used to assist in dehydration scenarios. Flea treatment can also be used. In some cases euthanasia may be the best option for treatment and to prevent further spreading.[21]
Prognosis
Untreated septicemic plague is almost always fatal, early treatment with antibiotics reduces the mortality rate to 11 percent. Death is almost inevitable if treatment is delayed more than about 24 hours, and some people may even die on the same day they present with the disease.[23][24]
Epidemiology
Plague cases were massively reduced during the second half of the 20th century, but outbreaks still occurred, especially in developing countries. Between 1954 and 1997, human plague was reported in 38 countries, making the disease a re-emerging threat to human health.[25] Between 1987 and 2001, 36,876 confirmed cases of plague with 2,847 deaths are reported to the World Health Organization.[26]
In 2015, Taylor Gaes, a 16-year-old in Larimer County in northern Colorado, contracted septicemic plague and subsequently died after being bitten by a flea that had bitten a rodent on his family's rural property.[27] Only three people in Colorado had contracted the bacteria in the previous thirty years.[28]
History

Septicemic plague was the least common of the three plague varieties that occurred during the Black Death from 1348 to 1350[30], the other two being bubonic plague and pneumonic plague.
Like the others, septicemic plague spread from the East through trade routes (Silk road) on the Black Sea and down to the Mediterranean Sea.[31] Much research has been done to ascertain what carrier was responsible for the transmission of the plague during this time period[32]
Major port cities and trade centers such as Venice and Florence were hit the hardest. [33][34]
The massive loss of working population in Europe following the Black Death, resulting in increased economic bargaining power of the serf labour force, was a major precipitating factor for the Peasants' Revolt of 1381.[35][36][37]
Other animals

Septicemic plague is a zoonosis,[38] a disease that generally is acquired by humans from animals, such as rodents and carnivores. Goats, sheep and camels also may carry the bacteria.. Areas west of the Great Plains of North America are one region where plague-infected animals commonly occur; plague-infested animals are found in many other countries as well.[39]
Animals that commonly carry plague bacteria are largely rodents and Leporidae, but carnivores sometimes also become infected by their prey. Prey animals are not immune to the disease, and outbreaks of various strains of plague, such as sylvatic plague, have on occasion devastated populations of black-tailed prairie dogs and black-footed ferrets.[12]
Plague has been active in black-tailed prairie dog populations since the 1960s. In the United States outbreaks only occur in the western States and they are devastating, with mortality rates near 100% because the animals have no immunity to the plague. Survivors are the ones that happened not to become infected and colonies that recover from a plague outbreak remain at risk.[40]
Because black-footed ferrets prey on black-tailed prairie dogs, wild ferret populations also fall victim to sylvatic plague. An outbreak can kill nearly 100% of ferrets in a population, and surviving ferrets commonly face starvation because the prairie dogs are their main prey. Spray-and-vaccinate campaigns have aimed at preventing the spread of the plague among these animals.[41]
Similar septicemic problems occur in many countries across the world, especially in developing countries where spending on health systems is low due to their economy[42]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Plague - Symptoms and Causes", Mayo Clinic, archived from the original on May 31, 2013, retrieved May 20, 2022
- ↑ Plague: Medline Plus Encyclopedia, National Library of Medicine, archived from the original on July 5, 2016, retrieved May 20, 2022
- ↑ Li, Min; Song, Yajun; Li, Bei; Wang, Zuyun; Yang, Ronghai; Jiang, Lingxiao; Yang, Ruifu (September 2005). "Asymptomatic Yersinia pestis Infection, China". Emerging Infectious Diseases. 11 (9): 1494–1496. doi:10.3201/eid1109.041147. PMC 3310611. PMID 16673521.
- ↑ Barbieri, R.; Signoli, M.; Chevé, D.; Costedoat, C.; Tzortzis, S.; Aboudharam, G.; Raoult, D.; Drancourt, M. (16 December 2020). "Yersinia pestis: the Natural History of Plague". Clinical Microbiology Reviews. 34 (1): e00044–19. doi:10.1128/CMR.00044-19. ISSN 1098-6618. PMC 7920731. PMID 33298527.
- ↑ 5.0 5.1 5.2 "Plague - Symptoms, Causes, Treatment | NORD". rarediseases.org (in español). Archived from the original on 2 July 2023. Retrieved 17 June 2023.
- ↑ "Plague: Background, Pathophysiology, Epidemiology". Medscape. 16 October 2021. Archived from the original on 24 June 2022. Retrieved 1 July 2023.
- ↑ "Blood Clotting Disorders - Disseminated Intravascular Coagulation (DIC) | NHLBI, NIH". www.nhlbi.nih.gov. 24 March 2022. Archived from the original on 20 April 2023. Retrieved 20 June 2023.
- ↑ Costello, Ryan A.; Nehring, Sara M. (2023). "Disseminated Intravascular Coagulation". StatPearls. StatPearls Publishing. Archived from the original on 14 July 2022. Retrieved 26 June 2023.
- ↑ "Human Plague Transmission from person to person" (PDF). CDC.gov. Archived (PDF) from the original on 6 March 2023. Retrieved 22 June 2023.
- ↑ Eldridge, B. F.; Edman, J. D. (6 December 2012). Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods. Springer Science & Business Media. p. 390. ISBN 978-94-007-1009-2. Archived from the original on 2 July 2023. Retrieved 24 June 2023.
- ↑ Wobeser, Gary A. (2006). Essentials of Disease in Wild Animals. Oxford: Blackwell Publishing. ISBN 978-0-8138-0589-4.
- ↑ 12.0 12.1 Ecology and Transmission, CDC, archived from the original on 2015-08-22, retrieved 2013-11-14
- ↑ Lathem WW, Price PA, Miller VL, Goldman WE (January 2007). "A plasminogen-activating protease specifically controls the development of primary pneumonic plague". Science. 315 (5811): 509–13. Bibcode:2007Sci...315..509L. doi:10.1126/science.1137195. PMID 17255510. S2CID 39881239.
- ↑ Collins, Frank M. (1996). "Pasteurella, Yersinia, and Francisella". Medical Microbiology (4th ed.). University of Texas Medical Branch at Galveston. ISBN 978-0-9631172-1-2. Archived from the original on 2021-05-06. Retrieved 16 June 2023.
- ↑ Salyers AA, Whitt DD (2002). Bacterial Pathogenesis: A Molecular Approach (2nd ed.). ASM Press. pp. 207–212.
- ↑ Plague, NYMedicalCenter, archived from the original on 2013-10-23, retrieved 2013-10-22
- ↑ "Diagnosis and treatment of plague | CDC". Centers for Disease Control and Prevention. 26 November 2019. Archived from the original on 5 April 2022. Retrieved 18 June 2023.
- ↑ 18.0 18.1 Yang, Ruifu (January 2018). "Plague: Recognition, Treatment, and Prevention". Journal of Clinical Microbiology. 56 (1): e01519-17. doi:10.1128/JCM.01519-17. PMC 5744195. PMID 29070654.
- ↑ "Plague resources for clinicians | CDC". Centers for Disease Control and Prevention. 25 February 2022. Archived from the original on 21 August 2015. Retrieved 19 June 2023.
- ↑ "Plague and Other Yersinia Infections - Infections". MSD Manual Consumer Version. Archived from the original on 10 May 2023. Retrieved 18 June 2023.
- ↑ 21.0 21.1 21.2 Plague in Cats, petMD, archived from the original on 2018-06-22, retrieved 2013-10-22
- ↑ Nelson, Christina A.; Meaney-Delman, Dana; Fleck-Derderian, Shannon; Cooley, Katharine M.; Yu, Patricia A.; Mead, Paul S. (16 July 2021). "Antimicrobial Treatment and Prophylaxis of Plague: Recommendations for Naturally Acquired Infections and Bioterrorism Response". MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports. 70 (3): 1–27. doi:10.15585/mmwr.rr7003a1. ISSN 1545-8601. Archived from the original on 9 February 2023. Retrieved 23 June 2023.
- ↑ Dillard, Robert L.; Juergens, Andrew L. (2023). "Plague". StatPearls. StatPearls Publishing. PMID 31751045. Archived from the original on 31 March 2021. Retrieved 19 June 2023.
- ↑ "Plague FAQ | CDC". Centers for Disease Control and Prevention. 15 November 2021. Archived from the original on 20 June 2012. Retrieved 2 July 2023.
- ↑ Xu, Lei; Liu, Qiyong; Stige, Leif Chr.; Ben Ar, Tamara; Fang, Xiye; Chan, Kung-Sik; Wang, Shuchun; Stenseth, Nils Chr.; Zhang, Zhibin (2011). "Nonlinear effect of climate on plague during the third pandemic in China". Proceedings of the National Academy of Sciences. 108 (25): 10214–10219. Bibcode:2011PNAS..10810214X. doi:10.1073/pnas.1019486108. PMC 3121851. PMID 21646523.
- ↑ Shahraki, Abdolrazagh Hashemi; Carniel, Elizabeth; Mostafavi, Ehsan (2016). "Plague in Iran: its history and current status". Epidemiology and Health. 38: e2016033. doi:10.4178/epih.e2016033. PMC 5037359. PMID 27457063.
- ↑ "Taylor Gaes dead: Plague kills Colorado high school student". NewsComAu. 2015-06-21. Archived from the original on 2015-09-20. Retrieved 2023-01-15.
- ↑ Peter Holley (21 June 2015). "Star teenage athlete dies after flu symptoms turn out to be plague". Washington Post. Archived from the original on 16 June 2018. Retrieved 15 January 2023.
- ↑ Schmid, Boris V.; Büntgen, Ulf; Easterday, W. Ryan; Ginzler, Christian; Walløe, Lars; Bramanti, Barbara; Stenseth, Nils Chr. (10 March 2015). "Climate-driven introduction of the Black Death and successive plague reintroductions into Europe". Proceedings of the National Academy of Sciences. 112 (10): 3020–3025. Bibcode:2015PNAS..112.3020S. doi:10.1073/pnas.1412887112. ISSN 0027-8424. PMC 4364181. PMID 25713390.
- ↑ History Learning Site - Black Death of 1348 to 1350, History Learning Site, archived from the original on 2009-05-23, retrieved 2011-06-06
- ↑ Huremović, Damir (2019). "Brief History of Pandemics (Pandemics Throughout History)". Psychiatry of Pandemics: 7–35. doi:10.1007/978-3-030-15346-5_2. ISBN 978-3-030-15345-8. PMC 7123574.
- ↑ Green, Monica H. (2015). "Taking "Pandemic" Seriously: Making the Black Death Global". The Medieval Globe. 1 (1): 27–61. doi:10.17302/tmg.1-1.3. ISSN 2377-3553. Archived from the original on 9 July 2022. Retrieved 3 July 2023.
- ↑ Stub, Sara (24 April 2020). "Venice's Black Death and the Dawn of Quarantine". SAPIENS. Archived from the original on 2 June 2023. Retrieved 29 June 2023.
- ↑ Tognotti, Eugenia (2013). "Lessons from the History of Quarantine, from Plague to Influenza A". Emerging Infectious Diseases. 19 (2): 254–259. doi:10.3201/eid1902.120312. ISSN 1080-6040. Archived from the original on 20 June 2023. Retrieved 29 June 2023.
- ↑ Ormrod, W. M. (1990). "The Peasants' Revolt and the Government of England". Journal of British Studies. 29 (1): 1–30. doi:10.1086/385947. ISSN 0021-9371. JSTOR 175483. S2CID 146331057. Archived from the original on 21 May 2021. Retrieved 20 June 2023.
- ↑ "800-year-old graves pinpoint where the Black Death began". 15 June 2022. doi:10.1126/science.add5045. Archived from the original on 16 June 2022. Retrieved 23 June 2023.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Villeins in the Middle Ages | Middle Ages". 31 May 2012. Archived from the original on 5 November 2018. Retrieved 5 November 2018.
- ↑ Plague facts, AVMA, archived from the original on 2013-10-29, retrieved 2013-10-22
- ↑ "Plague". American Veterinarian Medical Association. Archived from the original on 9 February 2023. Retrieved 20 June 2023.
- ↑ Plague and Black-tailed Prairie Dogs, FWS, archived from the original on 2017-05-29, retrieved 2013-11-14
- ↑ Plague Kills Many Prairie Dogs and Black-Footed Ferrets in Grasslands Near Badlands National Park, National Parks Traveler, archived from the original on 2017-02-21, retrieved 2013-11-14
- ↑ Butler, Thomas (October 2013). "Plague gives surprises in the first decade of the 21st century in the United States and worldwide". The American Journal of Tropical Medicine and Hygiene. 89 (4): 788–793. doi:10.4269/ajtmh.13-0191. ISSN 1476-1645. PMC 3795114. PMID 24043686.