Polidocanol
 | |
| Names | |
|---|---|
| Other names |
|
| |
| Clinical data | |
| Drug class | Nonionic detergent[1] |
| Main uses | Varicose veins[2] |
| Side effects | Local reaction, anaphylaxis, blood clots, tissue necrosis[2] |
| Routes of use | Injection into a vein |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Chemical and physical data | |
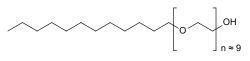
| Formula | C30H62O10 |
| Molar mass | 582.816 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Polidocanol, sold under the brand name Asclera, is a medication used to treat varicose veins including spider veins and reticular veins.[2] It may also be used for hemangiomas, and vascular malformations.[3] It is given by injection into the vein as part of sclerotherapy.[1]
Common side effects include a mild local reaction.[2] Other side effects may include anaphylaxis, blood clots, and tissue necrosis.[2] It is a nonionic detergent.[1]
Polidocanol was approved for medical use in the United States in 2010.[2] In the United States 2 ml of solution costs about 22 USD as of 2021.[4] It is also used topically in cosmetics.[5]
Medical uses
Sclerotherapy
Polidocanol is also used as a sclerosant, an irritant injected to treat varicose veins.[6]
The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue.[7][8] Polidocanol in the form of Varithena injected in the greater saphenous vein can cause the eruption of varicose and spider veins throughout the lower leg. This procedure should be done with caution and with the knowledge that the appearance of the leg may be forever compromised.
Mechanism
Polidocanol causes fibrosis inside varicose veins, occluding the lumen of the vessel, and reducing the appearance of the varicosity.
Names
It is available under the trade names Asclera, Aethoxysklerol,[9] and Varithena.[10]
References
- ↑ 1.0 1.1 1.2 "Polidocanol Monograph for Professionals". Drugs.com. Archived from the original on 28 May 2016. Retrieved 28 October 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "DailyMed - ASCLERA- polidocanol injection, solution". dailymed.nlm.nih.gov. Archived from the original on 24 March 2021. Retrieved 28 October 2021.
- ↑ Gao Z, Zhang Y, Li W, Shi C (January 2018). "Effectiveness and safety of polidocanol for the treatment of hemangiomas and vascular malformations: A meta-analysis". Dermatologic Therapy. 31 (1). doi:10.1111/dth.12568. PMID 29082587.
- ↑ "Asclera Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 28 October 2021.
- ↑ "ADDENDUM to the SCCP opinion on polidocanol (SCCP/1130/07)" (PDF). Archived (PDF) from the original on 14 June 2015. Retrieved 28 October 2021.
- ↑ Star P, Connor DE, Parsi K (April 2018). "Novel developments in foam sclerotherapy: Focus on Varithena® (polidocanol endovenous microfoam) in the management of varicose veins". Phlebology. 33 (3): 150–162. doi:10.1177/0268355516687864. PMID 28166694.
- ↑ Facts and Companies: Varicose Vein Treatment Approved Archived 2017-04-09 at the Wayback Machine
- ↑ "Asclera Full Prescribing Information in Drug Reference Encyclopedia". Archived from the original on 2016-03-03. Retrieved 2010-04-11.
- ↑ Sclerotherapy Archived 2008-10-26 at the Wayback Machine, Laurence Z Rosenberg, MD, eMedicine.com
- ↑ "Varithena™ (polidocanol injectable foam) For Intravenous Use. Full Prescribing Information" (PDF). Biocompatibles, Inc. Archived from the original (PDF) on 4 August 2016. Retrieved 1 October 2015.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Drugs with non-standard legal status
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- Fatty alcohols
- Polyethers
- Polymers
- Antipruritics
- Vascular surgery
- Non-ionic surfactants
- RTT