Etelcalcetide
 | |
| Names | |
|---|---|
| Trade names | Parsabiv |
| Other names | Velcalcetide, telcalcetide, AMG-416, KAI-4169, ONO-5163 |
| |
| Clinical data | |
| Drug class | Calcimimetic[1] |
| Main uses | Secondary hyperparathyroidism in people on hemodialysis[2] |
| Side effects | Low calcium, muscle spasms, diarrhea, nausea[1] |
| Routes of use | Intravenous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Elimination half-life | 3–5 days in dialysis patients |
| Excretion | 60% in dialysate, 7% in urine and faeces |
| Chemical and physical data | |
| Formula | C38H73N21O10S2 |
| Molar mass | 1048.26 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etelcalcetide, sold under the brand name Parsabiv, is a medication used to treat secondary hyperparathyroidism in people undergoing hemodialysis.[2] It is unclear if it changes outcomes such as life expectancy and heart disease.[2] It is given by injection into a vein at the end of each dialysis session.[2]
Common side effects include low calcium, muscle spasms, diarrhea, and nausea.[1] Other side effects may include allergic reactions, heart failure, upper GI bleeding, and adynamic bone disease.[2] It is a calcimimetic and works by activating calcium-sensing receptors in the parathyroid gland.[1]
Etelcalcetide was approved for medical use in Europe in 2016 and the United States in 2017.[2][1] In the United Kingdom a dose of 5 mg three times per week costs the NHS about £330 for 4 weeks as of 2021.[3] In the United States this amount costs about 2,100 USD.[4]
Medical uses
Etelcalcetide is used for the treatment of secondary hyperparathyroidism in people with chronic kidney disease (CKD) on hemodialysis.[5] Hyperparathyroidism is the condition of elevated parathyroid hormone (PTH) levels and is often observed in people with CKD.[6]
Dosage
It is used at a dose of 5 mg three times per week.[1] The dose may be increase up to 15 mg three times per week.[2]
Pharmacodynamics
Mechanism of action
Etelcalcetide functions by binding to and activating the calcium-sensing receptor (CaSR) in the parathyroid gland as an allosteric activator, resulting in PTH reduction and suppression.[7]
Pharmacokinetics
Etelcalcetide functions in a first order elimination, with a half life of 19 hours.[8]
No interaction studies in humans were conducted. Studies in vitro showed no affinity of etelcalcetide to cytochrome P450 enzymes or common transport proteins. Therefore, no relevant pharmacokinetic interactions are expected.[5][9]
Side effects
Common side effects (in more than 10% of people) are nausea, vomiting, diarrhoea, muscle spasms, and hypocalcaemia (too low blood calcium levels). In clinical studies, the latter side effect was usually mild to moderate and without symptoms. An increase of the QT interval of more than 60 ms was detected in 1.2% of people receiving etelcalcetide.[5][9]
Due to the lower iPTH levels achieved by the use of this drug, it is possible that adynamic bone disease could occur at levels "below 100 pg/mL"[8]
Contraindications
The drug is contraindicated in people with blood serum calcium levels below the norm.[5][9]
Chemistry
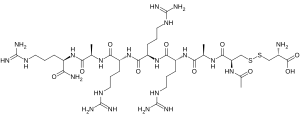
The substance is a peptide consisting mostly of D-amino acids instead of the common L-amino acids. More specifically, it is the disulfide of N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide with L-cysteine.[10]
History
Originally, Etelcalcetide was being developed by KAI Pharmaceuticals. After positive Phase II trials, Amgen acquired KAI for $315 Million.[11]
In 2011, KAI entered into agreement with Ono Pharmaceutical for production of etelcalcetide in Japan, the deal being worth ¥1 Billion.[8]
On 25 August 2015 Amgen Inc. announced its submission of a new drug application to the Food and Drug Administration for etelcalcetide.[7] The European Medicines Agency approved the drug in November 2016.[5]
In February 2017, the FDA approved Parsabiv for the treatment of secondary hyperparathyroidism.[12]
Society and culture
Parsabiv is currently owned by Amgen and Ono Pharmaceuticals in Japan.[13][8]
Research
Phase II trials found that Etelcalcetide was able to lower PTH levels in one cohort by -49% vs a 29% increase in the placebo group.[8] In another Phase II study "89% of patients experienced a C30% reduction in PTH and 56% achieved a PTH level of B300 pg/mL."[8]
In 2017, two Phase III trials found that using etelcalcetide showed greater symptom reduction compared to placebo.[14] Etelcalcetide was also able to lower PTH levels below 300pg/mL more often.[14]
Phase I Pediatric studies are planned for the USA and UK for etelcalcetide.[8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Parsabiv". Archived from the original on 21 June 2021. Retrieved 16 December 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Etelcalcetide Monograph for Professionals". Drugs.com. Archived from the original on 18 January 2021. Retrieved 16 December 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1094. ISBN 978-0857114105.
- ↑ "Parsabiv Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 17 April 2021. Retrieved 16 December 2021.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Parsabiv: EPAR – Product Information" (PDF). European Medicines Agency. 24 November 2016. Archived (PDF) from the original on 17 March 2018. Retrieved 22 December 2020.
- ↑ Wu Q, Lai X, Zhu Z, Hong Z, Dong X, Wang T, et al. (August 2015). "Evidence for Chronic Kidney Disease-Mineral and Bone Disorder Associated With Metabolic Pathway Changes". Medicine. 94 (32): e1273. doi:10.1097/MD.0000000000001273. PMC 4616673. PMID 26266360.
- ↑ 7.0 7.1 ""Amgen Submits New Drug Application For Novel Intravenous Calcimimetic Etelcalcetide (AMG 416)"". Archived from the original on 2015-10-09. Retrieved 2020-12-22.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Blair HA (December 2016). "Etelcalcetide: First Global Approval". Drugs. 76 (18): 1787–1792. doi:10.1007/s40265-016-0671-3. PMID 27900648. S2CID 45000617.
- ↑ 9.0 9.1 9.2 Haberfeld, H, ed. (2016). Austria-Codex (in Deutsch). Vienna: Österreichischer Apothekerverlag.
- ↑ "Etelcalcetide". ChemSpider. Archived from the original on 8 January 2017. Retrieved 7 January 2016.
- ↑ "Amgen - Investors - Press Release". investors.amgen.com. Archived from the original on 2021-10-31. Retrieved 2017-10-30.
- ↑ "Parsabiv (etelcalcetide) Injection". www.accessdata.fda.gov. Archived from the original on 2019-08-15. Retrieved 2017-10-30.
- ↑ "Parsabiv New FDA Drug Approval | CenterWatch". www.centerwatch.com. Archived from the original on 2017-10-29. Retrieved 2017-10-30.
- ↑ 14.0 14.1 "Drug and Device News". P & T. 42 (4): 223–265. April 2017. PMC 5358678. PMID 28381913.
External links
| Identifiers: |
|
|---|
- HSRIC Fact sheet 2014 Archived 2016-03-04 at the Wayback Machine
- Pages using duplicate arguments in template calls
- CS1 Deutsch-language sources (de)
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Webarchive template wayback links
- Nephrology procedures
- Systemic hormonal preparations
- Peptides
- RTT