User talk:QuackGuru/Sand 25
https://commons.wikimedia.org/wiki/Special:Watchlist
Unused sources
https://pubmed.ncbi.nlm.nih.gov/?linkname=pubmed_pubmed_citedin&from_uid=31252671
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9689130/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9232181/
[1] [2] JUUL and Combusted Cigarettes Comparably Impair Endothelial Function
References
- ↑ Aoki, Yayoi; Ikeda, Tomoya; Tani, Naoto; Shida, Alissa; Oritani, Shigeki; Ishikawa, Takaki (January 2020). "Evaluation of the distribution of nicotine intravenous injection: an adult autopsy case report with a review of literature". International Journal of Legal Medicine. 134 (1): 243–249. doi:10.1007/s00414-019-02035-y. PMC 6949309. PMID 30955048.
 This article incorporates text by Yayoi Aoki, Tomoya Ikeda, Naoto Tani, Alissa Shida, Shigeki Oritani, and Takaki Ishikawa available under the CC BY 4.0 license.
This article incorporates text by Yayoi Aoki, Tomoya Ikeda, Naoto Tani, Alissa Shida, Shigeki Oritani, and Takaki Ishikawa available under the CC BY 4.0 license.
Common recreational drugs
https://en.wikipedia.org/wiki/Recreational_drug_use#Common_recreational_drugs
https://en.wikipedia.org/w/index.php?title=Recreational_drug_use&diff=1182503121&oldid=1182432866
- Nicotine: Nicotine can be inhaled using an electronic cigarette.[1] A large proportion of e-cigarette use is used for recreational puroposes.[1] Most e-cigarette liquids contain nicotine, but the level of nicotine varies depending on user-preference and manufacturers.[2] Nicotine is highly addictive,[3][4][5] comparable to heroin or cocaine.[6] E-cigarettes are also being used to inhale MDMA, cocaine powder, crack cocaine, synthetic cathinones, mephedrone, α-PVP, synthetic cannabinoids, opioids, heroin, fentanyl, tryptamines, and ketamine.[7]
References
- ↑ 1.0 1.1 Rahman, Muhammad; Hann, Nicholas; Wilson, Andrew; Worrall-Carter, Linda (December 2014). "Electronic cigarettes: patterns of use, health effects, use in smoking cessation and regulatory issues". Tobacco Induced Diseases. 12 (1): 21. doi:10.1186/1617-9625-12-21. PMC 4350653. PMID 25745382.
- ↑ Burstyn, I (January 2014). "Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks". BMC Public Health. 14: 18. doi:10.1186/1471-2458-14-18. PMC 3937158. PMID 24406205.
- ↑ Grana, R; Benowitz, N; Glantz, SA (13 May 2014). "E-cigarettes: a scientific review". Circulation. 129 (19): 1972–86. doi:10.1161/circulationaha.114.007667. PMC 4018182. PMID 24821826.
- ↑ Holbrook, Bradley D. (June 2016). "The effects of nicotine on human fetal development". Birth Defects Research Part C: Embryo Today: Reviews. 108 (2): 181–92. doi:10.1002/bdrc.21128. ISSN 1542-975X. PMID 27297020.
- ↑ Siqueira, Lorena M. (January 2017). "Nicotine and Tobacco as Substances of Abuse in Children and Adolescents". Pediatrics. 139 (1): e20163436. doi:10.1542/peds.2016-3436. ISSN 0031-4005. PMID 27994114.
- ↑ Jenssen, Brian P.; Boykan, Rachel (February 2019). "Electronic Cigarettes and Youth in the United States: A Call to Action (at the Local, National and Global Levels)". Children. 6 (2): 30. doi:10.3390/children6020030. ISSN 2227-9067. PMC 6406299. PMID 30791645.
- ↑ Breitbarth, Andreas K.; Morgan, Jody; Jones, Alison L. (November 2018). "E-cigarettes—An unintended illicit drug delivery system". Drug and Alcohol Dependence. 192: 98–111. doi:10.1016/j.drugalcdep.2018.07.031. ISSN 0376-8716.
Electronic cigarettes
https://en.wikipedia.org/wiki/Flavored_tobacco#Electronic_cigarettes
Although the effects of the flavoring in e-cigarettes on human health have not been thoroughly studied, existing studies indicate that most flavoring can pose significant health risks if used for a long time, especially sweet flavoring.[1] Substances that have been identified by the American Flavor and Extract Manufacturers Association to be contained in e-cigarette flavorings are potential respiratory irritants or poisons, and a 2016 study also stated that the flavoring agents in e-cigarettes are an important factor in the production of toxic carbonyl and other substances.[1]
References
- ↑ 1.0 1.1 Zhang, Qing; Wen, Cai (15 May 2023). "The risk profile of electronic nicotine delivery systems, compared to traditional cigarettes, on oral disease: a review". Frontiers in Public Health. 11. doi:10.3389/fpubh.2023.1146949. PMC 10226679. PMID 37255760.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Qing Zhang and Cai Wen available under the CC BY 4.0 license.
This article incorporates text by Qing Zhang and Cai Wen available under the CC BY 4.0 license.
Passive smoking
https://en.wikipedia.org/wiki/Passive_smoking
Some prior investigators have emphasized that even very low levels of second-hand smoke exposure (serum cotinine concentrations below 0.1 ng/mL) can cause adverse effects on cognitive outcomes among children and this could be also attributed to the higher lead concentrations found in children exposed to second-hand smoke compared to those unexposed.[1]
References
- ↑ Mourino, Nerea; Ruano-Raviña, Alberto; Varela Lema, Leonor; Fernández, Esteve; López, María José; Santiago-Pérez, María Isolina; Rey-Brandariz, Julia; Giraldo-Osorio, Alexandra; Pérez-Ríos, Mónica (5 May 2022). "Serum cotinine cut-points for secondhand smoke exposure assessment in children under 5 years: A systemic review". PLOS ONE. 17 (5): e0267319. doi:10.1371/journal.pone.0267319. PMC 9070924. PMID 35511766.
 This article incorporates text by Nerea Mourino, Alberto Ruano-Raviña, Leonor Varela Lema, Esteve Fernández, María José López, María Isolina Santiago-Pérez, Julia Rey-Brandariz, Alexandra Giraldo-Osorio, and Mónica Pérez-Ríos available under the CC BY 4.0 license.
This article incorporates text by Nerea Mourino, Alberto Ruano-Raviña, Leonor Varela Lema, Esteve Fernández, María José López, María Isolina Santiago-Pérez, Julia Rey-Brandariz, Alexandra Giraldo-Osorio, and Mónica Pérez-Ríos available under the CC BY 4.0 license.
Disagreement among researchers
https://en.wikipedia.org/wiki/Tobacco_harm_reduction
Some researchers, particularly those supporting tobacco harm reduction, hold the position that "most of the harm caused by tobacco use is derived from exposure to combustion products of tobacco".[1] Others disagree, "the relative contributions of nicotine versus non-nicotine components of TC [tobacco cigarette] smoke are unknown".[1] The effects of inhaled nicotine are difficult to isolate from the smoke constituents (oral nicotine delivery has been studied) and "understanding the role of nicotine in cardiopulmonary disease is extraordinarily difficult".[1] Research has shown that nicotine activates the sympathetic nervous system, constricting coronary arteries, reducing coronary blood flow reserve, and causing transient increases in heart rate, blood pressure, and myocardial contractability.[1]
References
- ↑ 1.0 1.1 1.2 1.3 La Rosa, Giusy; Vernooij, Robin; Qureshi, Maria; Polosa, Riccardo; O’Leary, Renée (April 2023). "Clinical testing of the cardiovascular effects of e-cigarette substitution for smoking: a living systematic review". Internal and Emergency Medicine. 18 (3): 917–928. doi:10.1007/s11739-022-03161-z. PMC 10081981. PMID 36609804.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Giusy La Rosa, Robin Vernooij, Maria Qureshi, Riccardo Polosa, and Renée O’Leary available under the CC BY 4.0 license.
This article incorporates text by Giusy La Rosa, Robin Vernooij, Maria Qureshi, Riccardo Polosa, and Renée O’Leary available under the CC BY 4.0 license.
Smoking
Electronic cigarettes are handheld electronic devices that simulate the feeling of tobacco smoking.[1] Daily long-term use of high voltage (5.0 V) e-cigarettes may generate formaldehyde-forming chemicals at a greater level than smoking, which was determined to be a lifetime cancer risk of approximately 5 to 15 times greater than smoking.[2] However, the overall safety and long-term health effects of electronic cigarettes is still uncertain.[3]
https://en.wikipedia.org/wiki/Causes_of_cancer#Smoking Add citation: Electronic cigarettes or e-cigarettes are handheld electronic devices that simulate the action of tobacco smoking.[1]
References
- ↑ 1.0 1.1 Caponnetto, Pasquale; Campagna, Davide; Papale, Gabriella; Russo, Cristina; Polosa, Riccardo (2012). "The emerging phenomenon of electronic cigarettes". Expert Review of Respiratory Medicine. 6 (1): 63–74. doi:10.1586/ers.11.92. ISSN 1747-6348. PMID 22283580.
- ↑ Cooke, Andrew; Fergeson, Jennifer; Bulkhi, Adeeb; Casale, Thomas B. (July 2015). "The Electronic Cigarette: The Good, the Bad, and the Ugly". The Journal of Allergy and Clinical Immunology: In Practice. 3 (4): 498–505. doi:10.1016/j.jaip.2015.05.022. ISSN 2213-2198. PMID 26164573.
- ↑ Ebbert, Jon O.; Agunwamba, Amenah A.; Rutten, Lila J. (January 2015). "Counseling patients on the use of electronic cigarettes". Mayo Clinic Proceedings. 90 (1): 128–134. doi:10.1016/j.mayocp.2014.11.004. ISSN 1942-5546. PMID 25572196.
Redirect to Health effects of electronic cigarettes
Health effects of electronic cigarettes https://en.wikipedia.org/w/index.php?title=Health_risks_of_vaping&redirect=no
Useless redirects
https://en.wikipedia.org/w/index.php?title=Electronic_Cigarette_Association&redirect=no
COVID-19
https://en.wikipedia.org/wiki/Smoking_cessation
Cessation of smoking is likely to decrease the risk of COVID-19 as well as the likelihood of developing more severe complications.[1]
References
- ↑ Kashyap, Vivek K.; Dhasmana, Anupam; Massey, Andrew; Kotnala, Sudhir; Zafar, Nadeem; Jaggi, Meena; Yallapu, Murali M.; Chauhan, Subhash C. (9 September 2020). "Smoking and COVID-19: Adding Fuel to the Flame". International Journal of Molecular Sciences. 21 (18): 6581. doi:10.3390/ijms21186581. PMC 7555793. PMID 32916821.
 This article incorporates text by Vivek K. Kashyap, Anupam Dhasmana, Andrew Massey, Sudhir Kotnala, Nadeem Zafar, Meena Jaggi, Murali M. Yallapu, and Subhash C. Chauhan available under the CC BY 4.0 license.
This article incorporates text by Vivek K. Kashyap, Anupam Dhasmana, Andrew Massey, Sudhir Kotnala, Nadeem Zafar, Meena Jaggi, Murali M. Yallapu, and Subhash C. Chauhan available under the CC BY 4.0 license.
Philip Morris International
Advocacy groups that are indirectly funded by Philip Morris International such as the Foundation for a Smoke-Free World are contradicting public health officials, who say smoking puts individuals at a greater risk of experiencing serious complications from COVID-19.[1]
Philip Morris International remove ref from first sentence. Possible source.[3]
List of vaping bans in the United States
https://en.wikipedia.org/wiki/List_of_vaping_bans_in_the_United_States
E-cigarettes were initially advertised as a form of tobacco that could circumvent existing smoke-free legislation, with initial confusion as to whether existing smoke-free legislations also apply to e-cigarettes.[2] Increasingly as of 2019, smoke-free legislations banning combustible tobacco cigarette smoking in indoor public places have been amended to expand their coverage to e-cigarettes.[2] Many exceptions exist.[2] For instance, vaping is allowed in vape shops and also in venues that hold vaping conventions (even if the use of e-cigarettes is banned in those venues during other events).[2]
The Centers for Disease Control and Prevention, World Health Organization, and the American Heart Association have significant reservations in opposition to vaping and came to the conclusion that there are significant health risks related to their use.[3]
https://en.wikipedia.org/w/index.php?title=List_of_vaping_bans_in_the_United_States&diff=prev&oldid=1045622966 Needs a revert.
Vaping
https://en.wikipedia.org/wiki/Wikipedia:Contents/Health_and_fitness Add Vaping
https://www.cdph.ca.gov/Programs/CCDPHP/DCDIC/CTCB/Pages/ElectronicSmokingDevices.aspx
E-liquids contain nicotine in varying strengths.[4]
References
- ↑ {{cite news|url=https://www.bnnbloomberg.ca/philip-morris-money-is-funding-pro-vaping-coronavirus-spin-1.1422973%7Ctitle=Philip Morris Money Is Funding Pro-Vaping Virus Spin|last1=Kary|first1=Tiffany|agency=BNN Bloomberg|publisher=[[Bloomberg News]|date=21 April 2020}}
- ↑ 2.0 2.1 2.2 2.3 Jenssen, Brian; Boykan, Rachel (2019). "Electronic Cigarettes and Youth in the United States: A Call to Action (at the Local, National and Global Levels)". Children. 6 (2): 30. doi:10.3390/children6020030}. ISSN 2227-9067. PMC 6406299. PMID 30791645.
 This article incorporates text by Brian P. Jenssen1 and Rachel Boykan2 available under the CC BY 4.0 license.
This article incorporates text by Brian P. Jenssen1 and Rachel Boykan2 available under the CC BY 4.0 license.
- ↑ Kuntic, Marin; Hahad, Omar; Daiber, Andreas; Münzel, Thomas (2020). "Could E-cigarette vaping contribute to heart disease?". Expert Review of Respiratory Medicine. 14 (11): 1131–1139. doi:10.1080/17476348.2020.1807332. ISSN 1747-6348. PMID 32757856.
- ↑ Goniewicz, M. L.; Kuma, T.; Gawron, M.; Knysak, J.; Kosmider, L. (2012). "Nicotine Levels in Electronic Cigarettes". Nicotine & Tobacco Research. 15 (1): 158–166. doi:10.1093/ntr/nts103. ISSN 1462-2203.
https://www.flavorshookkids.org/ is part of the California Department of Public Health. The YouTube videos appear to be in the public domain. Therefore, the videos can be uploaded into one video. It was launched by the California Department of Public Health. https://www.vapeoutbreak.org/
References
https://ash.org/vaping-study-2019/ The first long-term study on vaping.[6]
Cited by:
https://academic.oup.com/function/article/2/2/zqab004/6130820
References
- ↑ 1.0 1.1 1.2 1.3 Tarran, Robert; Barr, R Graham; Benowitz, Neal L; Bhatnagar, Aruni; Chu, Hong W; Dalton, Pamela; Doerschuk, Claire M; Drummond, M Bradley; Gold, Diane R; Goniewicz, Maciej L; Gross, Eric R; Hansel, Nadia N; Hopke, Philip K; Kloner, Robert A; Mikheev, Vladimir B; Neczypor, Evan W; Pinkerton, Kent E; Postow, Lisa; Rahman, Irfan; Samet, Jonathan M; Salathe, Matthias; Stoney, Catherine M; Tsao, Philip S; Widome, Rachel; Xia, Tian; Xiao, DaLiao; Wold, Loren E (2021). "E-Cigarettes and Cardiopulmonary Health". Function. 2 (2). doi:10.1093/function/zqab004. ISSN 2633-8823. PMC 7948134. PMID 33748758.
A 41-year-old man developed an acute lung injury within hours of switching from traditional cigarettes to e-cigarettes.[1] He had acquired the unlicensed product from a friend, with the primary ingredient being a nicotine-based oil.[1]
References
- ↑ 1.0 1.1 Deliwala, Smit; Sundus, Saira; Haykal, Tarek; Theophilus, Nikita; Bachuwa, Ghassan (April 2020). "E-cigarette, or Vaping, Product Use-associated Lung Injury (EVALI): Acute Lung Illness within Hours of Switching from Traditional to E-cigarettes". Cureus. doi:10.7759/cureus.7513. ISSN 2168-8184. PMC 7195202. PMID 32373415.
 This article incorporates text by Smit Deliwala, Saira Sundus, Tarek Haykal, Nikita Theophilus, and Ghassan Bachuwa2 available under the CC BY 3.0 license.
This article incorporates text by Smit Deliwala, Saira Sundus, Tarek Haykal, Nikita Theophilus, and Ghassan Bachuwa2 available under the CC BY 3.0 license.
Health and medicine
https://en.wikipedia.org/wiki/List_of_topics_characterized_as_pseudoscience#Health_and_medicine
- Electronic cigarettes are marketed as a lower health risk option to tobacco smoking,[1] but it is more dangerous in the short-term than smoking and there is a risk of death from their short-term use.[2] No long term vaping toxicological/safety studies have been done in humans; without these data, saying with certainty that e-cigarettes are safer than combustible cigarettes is impossible.[3] Disease caused by tobacco has a latency period of no less than 25 years.[4] Therefore, as of 2019, it will conservatively take two decades until firm conclusions from long-term studies on using e-cigarettes are published.[4] Proponents of e-cigarettes think that these devices contain merely "water vapour" in the e-cigarette aerosols, but this view is refuted by the evidence.[5]
References
- ↑ Grana, R; Benowitz, N; Glantz, SA (13 May 2014). "E-cigarettes: a scientific review". Circulation. 129 (19): 1972–86. doi:10.1161/circulationaha.114.007667. PMC 4018182. PMID 24821826.
- ↑ Bhatt, Jayesh Mahendra; Ramphul, Manisha; Bush, Andrew (2020). "An update on controversies in e-cigarettes". Paediatric Respiratory Reviews. 36: 75–86. doi:10.1016/j.prrv.2020.09.003. ISSN 1526-0542. PMC 7518964. PMID 33071065.
- ↑ Gotts, Jeffrey E; Jordt, Sven-Eric; McConnell, Rob; Tarran, Robert (30 September 2019). "What are the respiratory effects of e-cigarettes?". BMJ (Clinical research ed.). BMJ. 366: l5275. doi:10.1136/bmj.l5275. ISSN 1756-1833. PMC 7850161. PMID 31570493.
 This article incorporates text by Jeffrey E Gotts, Sven-Eric Jordt, Rob McConnel, and Robert Tarran available under the CC BY 4.0 license.
This article incorporates text by Jeffrey E Gotts, Sven-Eric Jordt, Rob McConnel, and Robert Tarran available under the CC BY 4.0 license.
- ↑ 4.0 4.1 Strongin, Robert M. (12 June 2019). "E-Cigarette Chemistry and Analytical Detection". Annual review of analytical chemistry (Palo Alto, Calif.). Annual Reviews. 12 (1): 23–39. doi:10.1146/annurev-anchem-061318-115329. ISSN 1936-1327. PMC 6565477. PMID 30848928.
- ↑ Kaur, Gagandeep; Pinkston, Rakeysha; Mclemore, Benathel; Dorsey, Waneene C.; Batra, Sanjay (2018). "Immunological and toxicological risk assessment of e-cigarettes". European Respiratory Review. 27 (147): 170119. doi:10.1183/16000617.0119-2017. ISSN 0905-9180. PMID 29491036.
https://pubmed.ncbi.nlm.nih.gov/31591243/ Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice
Many countries, including the UK, recommend nicotine replacement therapy for smoking cessation during pregnancy and 11% of UK pregnant smokers receive replacement therapy prescriptions.[1] Although replacement therapy provides nicotine without other toxic elements present in tobacco smoke, the ability for nicotine to cross the placenta and concentrate in fetal blood and amniotic fluid leads to concerns that nicotine within replacement therapy may cause fetal harm.[1]
References
- ↑ 1.0 1.1 Phillips, Lucy; Thomson, Ross; Coleman-Haynes, Tom; Cooper, Sue; Naughton, Felix; Mcdaid, Lisa; Emery, Joanne; Coleman, Tim (3 February 2023). "Developing a taxonomy to describe offspring outcomes in studies involving pregnant mammals' exposure to non-tobacco nicotine: A systematic scoping review". PLOS ONE. 18 (2): e0280805. doi:10.1371/journal.pone.0280805. PMID 36735735.
 This article incorporates text by Lucy Phillips, Ross Thomson, Tom Coleman-Haynes, Sue Cooper, Felix Naughton, Lisa Mcdaid, Joanne Emery, Tim Coleman available under the CC BY 4.0 license.
This article incorporates text by Lucy Phillips, Ross Thomson, Tom Coleman-Haynes, Sue Cooper, Felix Naughton, Lisa Mcdaid, Joanne Emery, Tim Coleman available under the CC BY 4.0 license.
A 2019 study showed that mice exposed to nicotine delivered by means of electronic cigarette aerosol develop lung adenocarcinoma.[1] This suggests the need for caution when using nicotine replacement therapies and e-cigarettes.[1]
References
- ↑ 1.0 1.1 Pucci, Susanna; Zoli, Michele; Clementi, Francesco; Gotti, Cecilia (21 January 2022). "α9-Containing Nicotinic Receptors in Cancer". Frontiers in Cellular Neuroscience. 15: 805123. doi:10.3389/fncel.2021.805123.
 This article incorporates text by Susanna Pucci, Michele Zoli, Francesco Clementi, and Cecilia Gotti available under the CC BY 4.0 license.
This article incorporates text by Susanna Pucci, Michele Zoli, Francesco Clementi, and Cecilia Gotti available under the CC BY 4.0 license.
https://tobaccotactics.org/wiki/coehar/
https://en.wikipedia.org/wiki/Safety_of_electronic_cigarettes
https://en.wikipedia.org/wiki/Health_effects_of_electronic_cigarettes https://en.wikipedia.org/w/index.php?title=Health_effects_of_electronic_cigarettes&action=history&offset=&limit=500
Smokeless tobacco
https://en.wikipedia.org/wiki/Smokeless_tobacco#cite_ref-HollidayCampbell2019_23-0 At the end of the first paragraph.
Add: The International Agency for Research on Cancer does not consider nicotine to be a carcinogen, though several studies demonstrate it is carcinogenic.[1]
References
- ↑ Chaturvedi, Pankaj; Mishra, Aseem; Datta, Sourav; Sinukumar, Snita; Joshi, Poonam; Garg, Apurva (2015). "Harmful effects of nicotine". Indian Journal of Medical and Paediatric Oncology. 36 (1): 24. doi:10.4103/0971-5851.151771. ISSN 0971-5851. PMC 4363846. PMID 25810571.
https://en.wikipedia.org/wiki/Smokeless_tobacco
When you chew smokeless tobacco, the addictive chemical nicotine is absorbed through the tissue in your mouth, and other chemicals such as lead, formaldehyde, and carcinogens, like cadmium and arsenic, are also released.[1]
- Smokeless tobacco-related media
-
Smokeless tobacco is not safe[1]
-
Smokeless tobacco contains chemicals linked to cancer[1]
-
Smokeless tobacco may be harmful to gums[1]
-
Smokeless tobacco may cause tooth loss[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Dip, Chew, Snuff, Snus: "Smokeless" Doesn't Mean "Safe"". United States Food and Drug Administration. 16 May 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Change from Possible to Potential
https://mdwiki.org/wiki/File:Side_effects_of_nicotine.png https://commons.wikimedia.org/wiki/File:Side_effects_of_nicotine.png
https://mdwiki.org/wiki/File:Adverse_effects_of_vaping_(raster).png https://commons.wikimedia.org/wiki/File:Adverse_effects_of_vaping_(raster).png
Vail v. Juul Labs, Inc.
https://en.wikipedia.org/wiki/Vail_v._Juul_Labs,_Inc.
personal injury,[1] product liability,[1] wrongful death claim[2]
References
Vuse
https://en.wikipedia.org/wiki/Vuse
On May 12, the US FDA issued decisions on several Vuse Vibe and Vuse Ciro e-cigarette products, including the authorization of six new tobacco products through the Premarket Tobacco Product Application (PMTA) pathway.[1] The US FDA issued marketing granted orders (MGO) to R.J. Reynolds Vapor Company for its Vuse Vibe e-cigarette device and accompanying tobacco-flavored closed e-liquid pod, as well as for its Vuse Ciro e-cigarette device and accompanying tobacco-flavored closed e-liquid pod.[1] For each device, two versions of the Power Units were authorized to reflect different battery manufacturers described in the company’s applications.[1] In total, In total, the products receiving MGOs include: two Vuse Vibe Power Units, Vuse Vibe Tank Original 3.0%, 2 Vuse Ciro Power Units, and Vuse Ciro Cartridge Original 1.5%.[1]
This authorization allows these products to be legally marketed in the US.[1] While this action permits these specific products to be sold in the U.S., it does not mean these products are safe nor are they "FDA approved."[1] All tobacco products are harmful and potentially addictive. Those who do not use tobacco products shouldn’t start.[1]
The US FDA also issued marketing denial orders to R.J. Reynolds Vapor Company for multiple other Vuse Vibe and Vuse Ciro e-cigarette products.[1] Any of those products currently on the market must be removed or US FDA may take enforcement action.[1] Retailers should contact R.J. Reynolds Vapor Company with any questions about products in their inventory.[1]
Despite the US FDA's approval for Vuse products to reduce the amount of traditional cigarette use in adults since e-cigs, such as Vuse, would be readily marketed and easy to access, the evidence for the effectiveness of e-cigs in smoking cessation is both preliminary and unclear. [2]
The American Lung Association gave a public response to this decision and expressed their disappointment that the US FDA is failing to meet the Tobacco Control Act's public health standard.[2] The American Lung Association has a firm stance against the use of e-cigs for any population, warning of the irreversible lung damage and lung disease that it can cause.[2] The results of the 2021 NYTS study show that 10% of high school students who regularly utilize e-cigs use Vuse as their regular brand, yet the US FDA continued with the approval of Vuse products.[2] The fruity, candy, or mint-flavored ENDS products that younger populations use are part of the tobacco industry's plan for continuing the youth vaping epidemic.[2] Even if younger populations are less likely to progress to using combustible cigarettes, as mentioned in the US FDA's rationale, the e-cigarettes they use still result in higher rates of nicotine addiction which poses a risk to adolescents' health
Even though cigarette use by younger populations has declined over the past few years, there has been an increased incidence of nicotine use from ENDS products, such as Vuse.[2] The adolescent brain systems are known to have high plasticity, and the unique effects of nicotine on this plasticity are continuing to be determined.[2] Some of the major effects are seen within the drug-reward axis, as there is a higher number and activity of nicotinic acetylcholine receptors in areas of the brain associated with reward and increased nicotine-induced dopamine release in limbic regions.[2] This drug-reward relationship leads to an increased incidence of nicotine addiction in adolescent teens, as well as behavioral changes in the rewarding effects of other abused drugs.[2]
As a relatively new product when compared to traditional combustible cigarettes, the adverse effects of e-cig use are still being discovered. The current literature suggests multi-system consequences, but the respiratory system is at most risk.[2]
Even though cigarette use by younger populations has declined over the past few years leading up to 2022, there has been an increased incidence of nicotine use from ENDS products, such as Vuse.[2] By April 2022, Vuse was at 35.1% and Juul was at 33.1% of the market in the US.[3] Vuse's lead increased to 39.7%, while Juul's market share in the US slumped to 28.1% by September 2022.[4]
Fundamentals of chemosensation
https://en.wikipedia.org/wiki/Taste#Basic_tastes

By the early twentieth century, numerous researchers had recognized combined inputs from the taste, smell, and touch systems give rise to integrated percepts when we eat or drink.[5] In 1982, Rozin remarked that the word "flavor" best captures the combination of oral and olfactory sensations we perceive with ingestion of most foods, at least in English.[5] Today, most neuroscientists, sensory psychologists, and sensory and consumer scientists define flavor as the unitary percept which coalesces from the integration of smell, taste, and chemesthesis in the orbitofrontal cortex.[5] Despite this broad consensus, there remains some degree of confusion around these terms, regarding their colloquial and technical usage, even within medical professionals, so each of the three sensory modalities that contribute to flavor will be briefly detailed here.[5]
Olfaction (smell) occurs when we sense volatile chemical messages from the environment (via the nares) or from the oral cavity (through the back of the throat).[5] Odor active volatiles (i.e., odorants) activate specialized G-protein Coupled Receptors expressed in olfactory sensory neurons (OSNs) found near the top of the nasal cavity.[5] When an odorant binds to specialized receptor proteins expressed on the surface of OSNs, it initiates a transduction cascade which converts the chemical signal into an electrical signal.[5] The ensuing action potential is carried by the axon of the olfactory neuron through the cribriform plate, where the axons synapse onto second-order neurons in the olfactory bulb.[5] Because cell bodies of the OSNs sit at the top of the nasal cavity, below the cribriform plate, they are easily damaged by pollutants, viruses and toxins (including tobacco smoke).[5] However, OSNs are continually replaced, roughly every 30 days, which preserves function despite such environmental insults.[5] In contrast to other senses, smell is a dual sensory modality: that is, it occurs either orthonasally or retronasally and this affects where we localize the percept.[5] Ecologically speaking, orthonasal olfaction is an external sense focused on objects and information in the environment, while retronasal olfaction is an internally focused sense where volatiles that reach the olfactory epithelium via the pharyx during chewing or swallowing are perceived as being present in the mouth.[5]
Gustation (taste) occurs when non-volatile chemical stimuli dissolve in saliva and contact specialized taste receptor cells (TRCs) found in the tongue, soft palate and throat.[5] Unlike the OSNs mentioned above, the TRCs are not neurons—rather, they are specialized epithelial cells which must communicate with neurons to project a signal centrally.[5] Taste aids organisms in perception of nutrients and toxins, driving ingestion via affective responses.[5] The widely accepted prototypical taste qualities are sweet, salty, sour, bitter, and savory/umami (the meaty taste of certain amino acids).[5] Non-sweet starch taste, fatty acid taste (oleogustus), metallic taste, and astringent may also be distinct taste qualities, but the case for each is less clear and their inclusion as distinct qualities is still actively debated.[5] Individuals vary widely in terms of taste perception, due in part to genetic variation.[5] Such differences are potentially important for nicotine research, and are discussed more below.[5]
Chemesthesis is the sensibility that results from chemical stimulation of somatosensory nerves; that is, it can be thought of as chemically initiated touch.[5] Chemesthetic stimuli have a range of perceptual qualities, including the tingling elicited by carbonation, the burn from chili peppers, the burn from horseradish, the mechanical buzzing from Sichuan Buttons, and best known to tobacco researchers, the cooling from menthol.[5] As chemesthetic stimuli are known to trigger cough reflexes, they have strong relevance to e-cigarettes, especially given the importance of irritation or throat hit to e-cigarette liking and appeal.[5] Extensive discussions of menthol as it relates to use of nicotine containing products are covered in detail elsewhere, so comments below will be restricted to specific aspects related to narrowly to chemosensation.[5]
Notably, the classical assumption that nicotine is itself bitter is almost certainly in error.[5] Rather, three distinct and complementary lines of evidence suggest nicotine gives rise to chemesthetic sensations, rather than bitterness per se.[5] First, in heterologous expression systems, nicotine does not activate any known bitter taste receptor, but it does activate TRPA1, a receptor activated by ligands like cinnamaldehyde or allyl isothiocyanate (AITC) that impart the pungency of cinnamon and wasabi, respectively.[5] Second, electrophysiology data from rats and psychophysical data from humans each indicate nicotine is a chemesthetic stimulus.[5] Third, close reading of very old literature suggests a widely cited 1959 source for the widespread claim that nicotine is bitter in turn leads back to an earlier paper from 1885.[5] Critically, if one reads the original source from 1885, the authors explicitly write "nicotine does not trigger a taste sensation," noting that if the concentration is increased, it produces "a stinging sensation, which is not, strictly speaking, a taste sensation, but tactile".[5] This caveat notwithstanding, combustible tobacco smoke certainly gives rise to bitter sensations from one of the hundreds of other compounds found in smoke, but strictly speaking it does not seem such bitterness can be directly attributable to nicotine.[5] Regarding e-cigarettes, participants report bitterness in multiple studies, but the source of this bitterness remains unknown.[5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "FDA Issues Marketing Decisions on Vuse Vibe and Vuse Ciro E-Cigarette Products". United States Food and Drug Administration. 12 May 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Mir, Mikael; Rauf, Ibtisam; Goksoy, Sarah; Khedr, Anwar; Jama, Abbas B; Mushtaq, Hisham; Jain, Nitesh K; Khan, Syed Anjum; Surani, Salim; Koritala, Thoyaja (25 May 2022). "Electronic Cigarettes: Are They Smoking Cessation Aids or Health Hazards?". Cureus. doi:10.7759/cureus.25330. PMC 9232181. PMID 35761921.
 This article incorporates text by Mikael Mir, Ibtisam Rauf, Sarah Goksoy, Anwar Khedr, Abbas B Jama, Hisham Mushtaq, Nitesh K Jain, Syed Anjum Khan, Salim Surani, and Thoyaja Koritala available under the CC BY 4.0 license.
This article incorporates text by Mikael Mir, Ibtisam Rauf, Sarah Goksoy, Anwar Khedr, Abbas B Jama, Hisham Mushtaq, Nitesh K Jain, Syed Anjum Khan, Salim Surani, and Thoyaja Koritala available under the CC BY 4.0 license.
- ↑ Craver, Richard (13 June 2022). "FDA issues another limited authorization of e-cigarette products". Winston-Salem Journal.
- ↑ Craver, Richard (20 September 2022). "Vuse expands e-cigarette market share lead over Juul to double digits". Winston-Salem Journal.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 5.27 5.28 5.29 5.30 5.31 5.32 5.33 5.34 5.35 5.36 5.37 5.38 5.39 5.40 5.41 Hayes, John E.; Baker, Allison N. (27 July 2022). "Flavor science in the context of research on electronic cigarettes". Frontiers in Neuroscience. 16. doi:10.3389/fnins.2022.918082. PMC 9365686. PMID 35968379.
 This article incorporates text by John E Hayes and Allison N Baker available under the CC BY 4.0 license.
This article incorporates text by John E Hayes and Allison N Baker available under the CC BY 4.0 license.
Blood-brain barrier
https://en.wikipedia.org/wiki/Cocaine

The World Drug Report estimates that worldwide, 18.8 million people used cocaine in 2014.[1] In 2016, the National Institute on Drug Abuse reported an age-adjusted cocaine-mediated death rate of 52.4% in the US.[1] Cocaine is a highly addictive stimulant that restricts dopamine and monoamine reuptake through dopamine transporter (DAT) antagonism.[1] Monoamine oxidase inhibition leads to imbalanced free-radical production, which generates oxidative stress and neuroinflammation.[1] Continuous cocaine administration has been shown to contribute to a 50% increase in blood-brain barrier (BBB) permeability, with a concomitant decrease in trans endothelial electrical resistance (TEER) due to basement membrane and neurovascular capillary disruption, due to up-regulated matrix metalloproteinase (MMP) and tumor necrosis factor (TNF-α) expression.[1] Moreover, TJ protein loss and alteration, specifically decreased JAM-2 and zonula occludens-1 (ZO-1) levels, are characteristic of cocaine transit across the BBB.[1] CCL2 (C-C motif chemokine ligand-2) and CCR2 (C-C motif chemokine receptor-2) expression upregulation has also been reported.[1] Cocaine use affects intercellular junctions and causes cell ruffling, which contributes to increased permeability and decreased TEER values across BBB monolayers.[1]
An alternate pathway for cocaine-induced BBB permeability alteration involves platelet-derived growth factor (PDGF) intermediates.[1] Cocaine binding to sigma receptors evokes a proteolytic signal cascade that initiates PDGF-B chain assembly, a fundamental intermediate for increased membrane permeability that inhibits store-operated calcium entry.[1] Moreover, cocaine binding to sigma receptors has been associated with dopamine uptake inhibition and enhanced dopamine release that neutralizes the effects of antibody reversal on increased PDGF expression.[1] In rats, chronic cocaine exposure has been shown to increase BBB permeability in the hippocampus and striatum, suggesting that the hippocampus could be affected by glial and cytokine migration without significant changes in cortical or cerebellar permeability.[1] Furthermore, it has been recently revealed that acute cocaine administration alters BBB permeability and may increase neurotoxicity in free-moving rats.[1]
Astrocytes have complex morphologies involving extensive processes that communicate within the neurovascular unit and maintain the BBB.[1] Cocaine exposure potentiates aberrant astroglial responses in cellular and animal models, which leads to loss of BBB integrity and function.[1] Other studies have reported cocaine-induced neuroinflammation and BBB disruption mediated by the activation of brain microglial cells to secrete several cytokines, chemokines, and other neurotoxic factors.[1] Cocaine upregulates these inflammatory mediators and cell adhesion molecules, including intercellular adhesion molecule-1, vascular cell adhesion molecule, and activated leukocyte cell adhesion molecule in the BBB endothelium.[1]
Previous in vitro findings have shown that exposure of pericytes to cocaine upregulates pro-inflammatory cytokines [TNF-α, interleukin (IL)-1β, and IL-6] in both intracellular and extracellular compartments.[1] In addition, cocaine activates the Src–PDGFR-β–NF-κB pathway, which enhances CXCL10 [chemokine (C-X-C motif) ligand-1] secretion.[1] This causes increased neuroinflammation in human brain vascular pericytes, which further leads to neurovascular unit disruption and immune cell transmigration across the BBB.[1]
Blood-brain barrier
https://en.wikipedia.org/wiki/Methamphetamine

Methamphetamine is a highly addictive and illicit psychostimulant and is the second most widely abused drug in the US.[1] It adversely affects brain homeostasis through blood-brain barrier (BBB) dysfunction and hyperthermia.[1] Its high lipophilicity allows for rapid and comprehensive transmigration across the BBB.[1] Methamphetamine binding to the DAT induces reversal transport of norepinephrine, serotonin (5HT), and dopamine, which causes their excessive release into the synapse.[1] Moreover, it inhibits monoamine reuptake that leads to post-synaptic cleft stimulation.[1] Chronic methamphetamine administration causes irreversible impairment of serotonin and dopamine transport into synaptic terminals in various brain regions, especially in the hippocampus.[1]
Various methamphetamine dosing paradigms significantly disturb endothelial TJ assembly by inducing downregulation, fragmentation, or redistribution of major TJ proteins, including claudin-5 and ZO-1, which are mediated by MMP-1 and MMP-9 peptidases.[1] This leads to reduced endothelial barrier tightness and increased BBB paracellular permeability.[1] Moreover, repeated intravenous methamphetamine administration downregulates TJ proteins, which causes glutathione depletion and increases endothelial reactive oxygen species (ROS) levels.[1] This triggers actin polymerization that possibly involves activation of actin-related protein 2/3 complex or myosin light chain kinase and its downstream target RhoA.[1] In mice, research has shown that methamphetamine-induced glucose transporter and uptake downregulation is an important causative factor for BBB integrity loss.[1] Further, methamphetamine reduces TJ protein expression, rearranges the F-actin cytoskeleton, and increases BBB permeability through Rho-associated protein kinase-dependent pathway activation in the frontal lobes and isolated primary microvascular endothelial cells.[1]
Other neurotoxicity mechanisms have also been suggested, including the methamphetamine-induced increase in reactive oxidative stress and ROS levels, which activate myosin light chain protein kinase, thereby reducing TJ protein expression.[1] Additionally, methamphetamine-induced TJ protein downregulation and resulting BBB integrity disruption may involve activation of NF-κB transcription and pro-inflammatory cytokines (TNF-α) in BBB endothelial cells.[1] Methamphetamine transit across the BBB damages the nucleus accumbens shell region and prefrontal cortex and causes hyperthermia, neuroinflammation, and brain edema.[1] Studies have reported methamphetamine-induced pericyte migration via sigma-1 receptor activation, p53 upregulated modulator of apoptosis expression, and downstream mitogen-activated protein kinase and Akt/PI3K pathways in C3H/10T1/2 cells, leading to BBB dysfunction.[1] Methamphetamine-activated microglia and astrocytes in the neurovascular unit may promote neurotoxicity and astroglial reactivity and induces BBB integrity loss.[1] In addition, methamphetamine increases the expression of the glial fibrillary acidic protein, σ1 receptors, TNF-α, IL-6, and IL-8 in mouse and rat astrocytes.[1] This leads to methamphetamine-induced inflammation in microglial cells where increased TNF-α release can activate BBB endothelium, which increases transmigration of circulating leukocytes through the leaky BBB.[1]
Blood-brain barrier
https://en.wikipedia.org/wiki/Morphine

Opioids are widely used analgesics that bind with opioid and/or toll-like receptors (TLR) in the CNS.[1] Transcellular solute and xenobiotic transport across the blood-brain barrier (BBB) is selectively controlled by the local influx and efflux transporters, including ATP-binding cassette (ABC), P-glycoprotein (P-gp, ABCB1), breast cancer resistance protein (ABCG2), multidrug resistance-associated proteins (ABCC) transporters, and solute carrier transporters.[1] Among the four central opioid receptor families [mu (μ), delta (δ), kappa (κ), and opioid receptor like-1 (ORL1) receptor], μ-opioid receptors are primarily responsible for the analgesic effects.[1] Microvascular endothelial cells have high affinity and specific opiate binding sites that mediate morphine’s effects on the CNS.[1]
Morphine exerts its effects by directly acting on the CNS with its illicit use leading to tolerance and drug dependence.[1] Drug transmigration is essential to psychological dependence.[1] Morphine alters BBB homeostasis and permeability through pro-inflammatory cytokine activity, intracellular calcium release dysregulation, and myosin light chain protein kinase activation, which results in ROS-mediated neurotoxicity.[1]
P-gp limits the net transport of several foreign substrates into the brain through active unidirectional efflux.[1] This transporter regulates foreign-agent pharmacokinetics in the brain by inhibiting or augmenting their movement across the BBB, which restrains morphine entry into the brain.[1] Moreover, P-gp attenuates morphine-induced migratory properties and transcriptional effects.[1] Acute morphine treatment inhibits P-gp expression, which increases morphine uptake in the brain, which modifies the acute analgesic and locomotive morphine effects and selectively alters critical transcriptional responses in the nucleus accumbens.[1] This indicates that the transporter system significantly contributes to mediating BBB integrity and permeability of carrier mediated transport.[1]
Blood-brain barrier
https://en.wikipedia.org/wiki/Heroin

There has been a rapid increase in opioid abuse in the US with approximately 580 new heroin users every day.[1] Deaths resulting from opiate overdose, including pain relievers and heroin, increased by 200% between 2000 and 2014.[1] Heroin can be reversibly metabolized into morphine; upon selective transmigration across the blood-brain barrier (BBB), heroin is transformed into morphine and metabolized into 6-monoacetylmorphine (6-MAM).[1] The superior heroin lipophilicity allows faster transit across the BBB than morphine.[1] The acetylation of both hydroxyl groups while synthesizing heroin increases its BBB penetration rate by 100-fold, which could contribute to its high addictive potential.[1] These addictive properties are regulated by the μ-opioid receptor (MOR), which mediates the rewarding effects of heroin.[1] A recent study reported that 6-MAM has a higher affinity for μ-opioid receptor G-protein activation than morphine.[1]
Heroin's effects indirectly involve its metabolites (morphine and 6-MAM) that act as substrates in P-gp membrane regulation.[1] Upon heroin transition into the brain, it has a higher synthesized concentration than morphine.[1] This suggests that the metabolite is the primary effector of the detrimental effects of heroin on the BBB.[1] In the extracellular brain fluid, these metabolites bind and activate MORs, which regulates crucial neurological automatic processes.[1] P-gp inhibition at the BBB acutely disrupts the BBB permeability and selectivity in the nucleus accumbens.[1] Moreover, increased levels of these metabolites in the brain downregulate TJ protein expression, especially ZO-1, which increases BBB permeability.[1] Contrastingly, there have been reports of increased JAM-2 TJ protein expression.[1]
Blood-brain barrier
https://en.wikipedia.org/wiki/Alcohol_and_health

Alcohol is a widely used recreational drug responsible for 5.3% of deaths worldwide.[1] In the US, there are 23 million alcohol addicts with 88,000 people dying from alcohol use disorder.[1] Alcohol acts on neurotransmitter receptors, including GABA, glutamate, and dopamine, with each receptor contributing to various physiologic effects, with chronic alcohol administration increasing tolerance and addiction.[1] Further, occasional alcohol consumption could lead to alcohol use disorder due to addiction and tolerance.[1] Regular and excessive alcohol consumption causes brain injury, white matter loss, reduced brain volume, and neuronal loss associated with the BBB.[1] Moreover, gray matter loss is positively correlated with years of alcohol abuse.[1] Chronic alcohol abuse induces neuroplastic changes and loss of neural circuit structure and strength.[1]
The brains of individuals with alcohol dependence have increased proinflammatory cytokines, chemokines, microglial markers, and inflammasome proteins.[1] Inflammatory cytokine and ROS activation contributes to BBB integrity disruption in TLR4-knockout mice.[1] Further, postmortem alcoholic brains have shown increased TLR2, TLR3, and TLR4 expression in the orbitofrontal cortex, which correlates with BBB integrity loss.[1] Moreover, they indicate that chronic alcohol intake increases TJ and neuroinflammatory protein (ERK1/2 and p-38) degradation, which may promote leukocyte brain infiltration.[1]
Brain microvascular endothelial cells (BMVEC) are interconnected with TJ to form a tight monolayer in the BBB.[1] Exposure of BMVEC to alcohol increases oxidative stress through myosin light chain and TJ protein phosphorylation.[1] This leads to decreased TEER and increased leukocyte migration across the BBB.[1] Further, alcohol induces BBB dysfunction and neuroinflammation through MMP-3/9 activation and angiogenesis (VEGF)-A/VEGFR-2) impairment in primary endothelial cells in the brain.[1] Ethanol (EtOH) disrupts BBB integrity via endothelial transient receptor potential (TRP) channels, which affects the intracellular Ca2+ and Mg2+ dynamics.[1] This increases endothelial permeability and alters inflammatory responses.[1] EtOH-mediated TRPM7 expression downregulation causes BBB dysfunction and endothelium integrity loss.[1] Overall, TRP channels are involved in alcohol-mediated BBB dysfunction.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 1.38 1.39 1.40 1.41 1.42 1.43 1.44 1.45 1.46 1.47 1.48 1.49 1.50 1.51 1.52 1.53 1.54 1.55 1.56 1.57 1.58 1.59 1.60 1.61 1.62 1.63 1.64 1.65 1.66 1.67 1.68 1.69 1.70 1.71 1.72 1.73 1.74 1.75 1.76 1.77 1.78 1.79 1.80 1.81 1.82 1.83 1.84 1.85 1.86 1.87 1.88 1.89 1.90 1.91 1.92 1.93 1.94 1.95 1.96 1.97 1.98 Pimentel, Emely; Sivalingam, Kalaiselvi; Doke, Mayur; Samikkannu, Thangavel (21 May 2020). "Effects of Drugs of Abuse on the Blood-Brain Barrier: A Brief Overview". Frontiers in Neuroscience. 14. doi:10.3389/fnins.2020.00513. PMC 7326150. PMID 32670001.
 This article incorporates text by Emely Pimentel, Kalaiselvi Sivalingam, Mayur Doke, and Thangavel Samikkannu available under the CC BY 4.0 license.
This article incorporates text by Emely Pimentel, Kalaiselvi Sivalingam, Mayur Doke, and Thangavel Samikkannu available under the CC BY 4.0 license.
COVID-19
https://en.wikipedia.org/wiki/Hookah#Health_effects
Another way of smoking is a hookah (shisha or waterpipe), a single- or multi-stemmed instrument typically used by multiple people simultaneously.[1] In the US, "hookah bars" have gained popularity in recent years with nearly 2.6 million people smoking hookah products and there also an estimated 100 million hookah users worldwide.[1] By virtue of their design, hookahs are ideal vectors for viral spreading and may escalate the risk for more severe COVID-19 infections through public use, complex cleaning requirements, and a cold-water reservoir, suitable for SARS-CoV-2 transmission.[1] In addition, hookah smoke contains some harmful chemicals that can damage the respiratory lining and predispose smokers to respiratory infection such as MERS-CoV.[1] Due to the risks of public health posed by transmission of SARS-CoV-2, some countries have already imposed restrictions on hookah use.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Kashyap, Vivek K.; Dhasmana, Anupam; Massey, Andrew; Kotnala, Sudhir; Zafar, Nadeem; Jaggi, Meena; Yallapu, Murali M.; Chauhan, Subhash C. (9 September 2020). "Smoking and COVID-19: Adding Fuel to the Flame". International Journal of Molecular Sciences. 21 (18): 6581. doi:10.3390/ijms21186581. PMC 7555793. PMID 32916821.
 This article incorporates text by Vivek K. Kashyap, Anupam Dhasmana, Andrew Massey, Sudhir Kotnala, Nadeem Zafar, Meena Jaggi, Murali M. Yallapu, and Subhash C. Chauhan available under the CC BY 4.0 license.
This article incorporates text by Vivek K. Kashyap, Anupam Dhasmana, Andrew Massey, Sudhir Kotnala, Nadeem Zafar, Meena Jaggi, Murali M. Yallapu, and Subhash C. Chauhan available under the CC BY 4.0 license.
Pulmonary effects
https://en.wikipedia.org/wiki/Effects_of_cannabis

Cannabis, as well as tobacco, contains a toxic combination of gases and other substances that can be injurious to the pulmonary system.[2] Cannabis smokers usually smoke fewer "joints" than tobacco smokers consume cigarettes; however, methods of cannabis smoking may place more cannabis particulate matter into the lungs than noted with typical cigarette smoking.[2] Those with cannabis dependence will continue to use it despite chronic cough, excessive sedation, or other marijuana-related problems.[2] Combining marijuana with tobacco leads to known tobacco-effects via second-hand smoke.[2]
Cannabis use can induce some bronchodilation but regular or heavy cannabis consumption can result in generalized airway inflammation with evidence of respiratory epithelial cell injury and damage to alveolar macrophages which can lead to pulmonary infection.[2] Sharing of cannabis water pipes has led to the development of pulmonary tuberculosis.[2] Smoking cannabis that contains fungal spores can result in pulmonary aspergillosis in those with immune-compromised conditions.[2]
There is a dose-related large airway dysfunction with hyperinflation and obstruction of airflow; one cannabis joint has been noted to be equivalent to 2.5–5 cigarettes in terms of this pulmonary dysfunction.[2] Macrophage injury can result in cytokine and nitric oxide impairment.[2] Smokers of cannabis are typically exposed to more carbon monoxide and tar than cigarette smokers; this effect is not related to the THC content.[2]
Heavy and/or chronic users of cannabis may have persistent cough, bronchitis (bullous) emphysema [chronic obstructive lung disease (COPD)], pulmonary dysplasia, pneumothorax, TB, and other respiratory infections.[2] Cannabis can lead to increased airway resistance and large airway inflammation though causal links to COPD or macroscopic emphysema remain controversial and unproven.[2] Smoking both tobacco and marijuana increases risks for abnormal tracheobronchial histopathology and COPD.[2]
In addition to the release of cannabinoids, smoking cannabis also generates a myriad of pyrogenic compounds, including carcinogens, mutagens, and teratogens, that have the potential to cause adverse health outcomes.[1] These compounds are similar to those found in cigarette smoke.[1] Cigarette smoke and cannabis smoke have 231 compounds in common, with 69 of these being toxic.[1] In contrast to cigarette smoke, the effects of cannabis smoke on the pulmonary system are much less well understood.[1] A major challenge is that many cannabis smokers also use tobacco products]; almost 90% of individuals who smoke cannabis also smoke tobacco cigarettes.[1] Moreover, there are differences in how people inhale cannabis smoke compared to tobacco smokers. Cannabis smokers take larger puffs, inhale more deeply, and hold their breath four times longer, which leads to a different deposition of particles and increased tar deposition.[1] Despite being in direct contact with inhaled compounds, the impact of cannabis smoke on pulmonary immunity remains poorly understood, with much of the information centered on assessment of immune cell recruitment.[1]
https://en.wikipedia.org/wiki/Effects_of_cannabis#Short-term_effects
Out of 266 people tested using delta-9-tTHC administered through an IV from 1997 until January 1, 2010, nausea and dizziness were the most commonly cited adverse effects.[3]
Microbial contamination
https://en.wikipedia.org/wiki/Cannabis_edible
Microbial contamination of cannabis-containing foods for instance can lead to foodborne illness, especially in persons with weak immune systems or other underlying health conditions.[4] In one study, researchers analyzed a variety of cannabis-infused food products and found that many were contaminated with high levels of bacteria including E. coli and Salmonella.[4] In other studies, a significant percentage of cannabis products tested were found to be contaminated with pesticides, mycotoxins, and heavy metals above the legal limit.[4] Another issue is the lack of standardized dosing guidelines for cannabis-containing foods.[4] Because the potency of these products can vary widely, it can be difficult for consumers to know how much of a particular product they should consume to achieve the desired effects without risking overconsumption.[4] This has led to instances of accidental overconsumption and adverse effects, particularly in the case of edibles, which can be deceivingly potent.[4] Another concern is the potential for cannabis-containing foods to be appealing to children and young people.[4] As these products become more widely available, there is a risk that they could be mistaken for regular food items and ingested by children, potentially leading to serious adverse effects.[4] A 2017 study found that the rate of emergency department visits related to cannabis-containing edibles increased significantly after legalization in Colorado. [4]
Packaging and labeling regulations
https://en.wikipedia.org/wiki/Cannabis_edible
Some jurisdictions have implemented packaging and labeling regulations for cannabis-containing products to make them less appealing to children.[4] In the US, for example, the US FDA requires that all cannabis-containing food products be labeled with the statement "Keep out of reach of children" and include a warning that the product contains cannabis.[4] Some US states such as Colorado, California, and Washington have gone further, requiring that products be packaged in child-resistant containers or that the packaging be opaque or non-descript to reduce their appeal to children.[4] Similarly, in Canada, the cannabis Act requires that all cannabis-containing products be packaged in child-resistant containers and display a standardized warning label that includes the THC content and other relevant information.[4] In addition, the act prohibits the use of branding and labeling that may appeal to children, such as cartoon characters or bright colors.[4] In Australia, cannabis-containing products must be packaged in opaque, child-resistant packaging and display warnings about the potential health risks associated with consumption.[4] In the Netherlands, all cannabis-containing products must be labeled with a warning that they are not intended for consumption by children or minors.[4]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Preteroti, Matthew; Wilson, Emily T.; Eidelman, David H.; Baglole, Carolyn J. (28 March 2023). "Modulation of pulmonary immune function by inhaled cannabis products and consequences for lung disease". Respiratory Research. 24 (1). doi:10.1186/s12931-023-02399-1. PMC 10043545. PMID 36978106.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Matthew Preteroti, Emily T. Wilson, David H. Eidelman, and Carolyn J. Baglole available under the CC BY 4.0 license.
This article incorporates text by Matthew Preteroti, Emily T. Wilson, David H. Eidelman, and Carolyn J. Baglole available under the CC BY 4.0 license.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Greydanus, Donald E.; Hawver, Elizabeth K.; Greydanus, Megan M.; Merrick, Joav (2013). "Marijuana: Current Concepts†". Frontiers in Public Health. 1. doi:10.3389/fpubh.2013.00042. PMC 3859982. PMID 24350211.
 This article incorporates text by Donald E Greydanus, Elizabeth K Hawver, Megan M Greydanus, and Joav Merrick available under the CC BY 4.0 license.
This article incorporates text by Donald E Greydanus, Elizabeth K Hawver, Megan M Greydanus, and Joav Merrick available under the CC BY 4.0 license.
- ↑ Carbuto, Michelle; Sewell, R. Andrew; Williams, Ashley; Forselius-Bielen, Kim; Braley, Gabriel; Elander, Jacqueline; Pittman, Brian; Schnakenberg, Ashley; Bhakta, Savita; Perry, Edward; Ranganathan, Mohini; D’Souza, Deepak Cyril (February 2012). "The safety of studies with intravenous Δ9-tetrahydrocannabinol in humans, with case histories". Psychopharmacology. 219 (3): 885–896. doi:10.1007/s00213-011-2417-y. PMID 21845389.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 Fordjour, Eric; Manful, Charles F.; Sey, Albert A.; Javed, Rabia; Pham, Thu Huong; Thomas, Raymond; Cheema, Mumtaz (15 June 2023). "Cannabis: a multifaceted plant with endless potentials". Frontiers in Pharmacology. 14. doi:10.3389/fphar.2023.1200269. PMC 10308385. PMID 37397476.
{{cite journal}}: Check|pmc=value (help) This article incorporates text by Eric Fordjour, Charles F. Manful, Albert A. Sey, Rabia Javed, Thu Huong Pham, Raymond Thomas, and Mumtaz Cheema available under the CC BY 4.0 license.
This article incorporates text by Eric Fordjour, Charles F. Manful, Albert A. Sey, Rabia Javed, Thu Huong Pham, Raymond Thomas, and Mumtaz Cheema available under the CC BY 4.0 license.
Environmental impact of electronic cigarettes
https://en.wikipedia.org/wiki/Environmental_impact_of_electronic_cigarettes
Change to Environmental impact of disposable electronic cigarettes
Systemic manifestation of COVID-19 infection
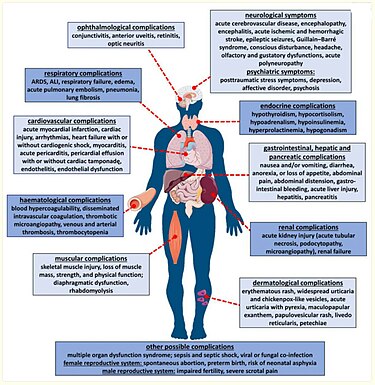
References
- ↑ Malinowska, Barbara; Baranowska-Kuczko, Marta; Kicman, Aleksandra; Schlicker, Eberhard (17 February 2021). "Opportunities, Challenges and Pitfalls of Using Cannabidiol as an Adjuvant Drug in COVID-19". International Journal of Molecular Sciences. 22 (4): 1986. doi:10.3390/ijms22041986. PMC 7922403. PMID 33671463.
 This article incorporates text by Barbara Malinowska, Marta Baranowska-Kuczko, Aleksandra Kicman, and Eberhard Schlicker available under the CC BY 4.0 license.
This article incorporates text by Barbara Malinowska, Marta Baranowska-Kuczko, Aleksandra Kicman, and Eberhard Schlicker available under the CC BY 4.0 license.
Category:Electronic cigarette aerosol carcinogens
A 2021 review states that, "Formaldehyde, acetaldehyde, acrolein, carcinogenic nitrosamines N'-nitrosonornicotine (NNN) and nicotine-derived nitrosamine ketones (NNK) were found in vapors of a variety of e-cigarette products and are all carcinogenic to humans."[1]
References
- ↑ Famiglietti, Amber; Memoli, Jessica Wang; Khaitan, Puja Gaur (2021). "Are electronic cigarettes and vaping effective tools for smoking cessation? Limited evidence on surgical outcomes: a narrative review". Journal of Thoracic Disease. 13 (1): 384–395. doi:10.21037/jtd-20-2529. ISSN 2072-1439. PMC 7867832. PMID 33569219.
Copyright violations
https://en.wikipedia.org/w/index.php?title=Cannabinoid_hyperemesis_syndrome&diff=next&oldid=840426931 Revision as of 20:39, 9 May 2018
https://mdwiki.org/w/index.php?title=Cannabinoid_hyperemesis_syndrome&diff=next&oldid=324008 Revision as of 20:39, 9 May 2018
See Table 6: "Severe cyclic vomiting usually accompanied by abdominal pain"[7] All the copied content is a copyright violation.



