Breast cancer chemotherapy
| Breast cancer chemotherapy | |
|---|---|
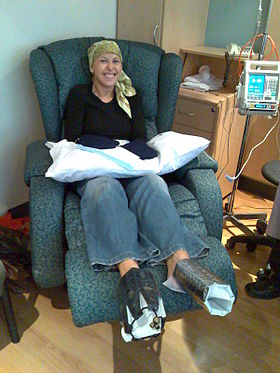 A woman being treated with docetaxel chemotherapy for breast cancer. Cold mittens and wine chilling devices are placed on her hands and feet to prevent deleterious effects on the nails. Similar strategies can be used to prevent hair loss. | |
| Specialty | oncology |
Breast cancer chemotherapy refers to the use of cytotoxic drugs (chemotherapy) in the treatment of breast cancer.
Types
There are three major types of chemotherapy.
- Neoadjuvant chemotherapy
- Neoadjuvant chemotherapy is given before surgery to slow the growth of a fast-growing cancer or to shrink the size of a larger breast cancer.[1]
- It is frequently used to treat locally advanced cancers, cancers that at the time of diagnosis are too large to be removed by surgery, which can then be removed with less extensive surgery.[2]
- Adjuvant chemotherapy
- Adjuvant chemotherapy is given after surgery to reduce the risk of recurrence. It is used to try to kill any cancer cells that might still exist and cannot be detected through imaging tests.[2]
- Palliative chemotherapy
- Palliative chemotherapy is used to control (but not cure) the cancer in settings in which the cancer has spread beyond the breast and localized lymph nodes. See metastatic breast cancer.
- Combined therapies
- These combine, for example, non-drug treatments with localized chemotherapy to limit toxicity and achieve better results.[3]
Regimens
Multiple chemotherapeutic agents may be used in combination to treat patients with breast cancer. Determining the appropriate regimen to use depends on many factors; such as, the character of the tumor, lymph node status, and the age and health of the patient.[3]
The following is a list of some of the commonly used adjuvant chemotherapy for breast cancer:
- CMF: cyclophosphamide, methotrexate, and 5-fluorouracil.[4]
- FAC (or CAF): 5-fluorouracil, doxorubicin, cyclophosphamide.[5]
- AC (or CA): Adriamycin (doxorubicin) and cyclophosphamide.[6]
- AC-Taxol: AC followed by paclitaxel (Taxol).[7]
- TAC: Taxotere (docetaxel), Adriamycin (doxorubicin), and cyclophosphamide.[8]
- FEC: 5-fluorouracil, epirubicin and cyclophosphamide.[9]
- AT: Adriamycin (doxorubicin) and Taxotere (docetaxel).[3]
Since chemotherapy affects the production of white blood cells, a granulocyte colony-stimulating factor (G-CSF) is sometimes administered along with chemotherapy. This has been shown to reduce, though not completely prevent, the rate of infection and low white cell count. Most adjuvant breast cancer chemotherapy regimens do not routinely require growth factor support except for those associated with a high incidence of bone marrow suppression and infection. These may include chemotherapy given in the dose dense fashion i.e. 2-weekly instead of 3-weekly or TAC chemotherapy (see above).[10]
In women with operable early breast cancer, taxane-containing adjuvant chemotherapy regimens have been found to improve overall survival and disease-free survival.[11]
Anthracylines
By conducting a meta-analysis of four large breast cancer trials including nearly 3,000 patients, the researchers have discovered that an abnormality on chromosome 17, called CEP17, is associated with a worse outcome for patients, but also that its presence is a highly significant indicator that the tumor will respond to anthracyclines.[12]
CEP17 is detected by a common and straightforward test (fluorescent in situ hybridisation or FISH), which is carried out routinely in breast cancer patients; it is used to test for the HER2 gene to see whether the women might benefit from the drug Herceptin. Assessment for CEP17, according to John Bartlett, professor of Molecular Pathology at the University of Edinburgh, could be easily carried out in the same FISH analysis as for HER2.[13]
Administration
For breast cancer, chemotherapy drugs are given into a vein (intravenously) or by mouth as tablets or capsules (orally).[14]
See also
References
- ^ "Chemotherapy". Breast Cancer Care. 2016-10-26. Retrieved 2018-04-20.
- ^ a b "Chemotherapy for Breast Cancer". www.cancer.org. Retrieved 2018-04-20.
- ^ a b c "Choosing a Chemotherapy Combination to Treat Breast Cancer | Breastcancer.org". Breastcancer.org. Retrieved 2018-04-20.
- ^ "Breakthrough - CMF (cyclophosphamide, methotrexate and 5-fluorouracil)". 2009-01-06. Archived from the original on 2009-01-06. Retrieved 2018-04-20.
- ^ "CAF". National Cancer Institute. 18 September 2009. Retrieved 2018-04-20.
- ^ "AC". National Cancer Institute. 22 April 2010. Retrieved 2018-04-20.
- ^ "AC-T". National Cancer Institute. 18 September 2009. Retrieved 2018-04-20.
- ^ "TAC". National Cancer Institute. 20 November 2013. Retrieved 2018-04-20.
- ^ "FEC". National Cancer Institute. 8 July 2011. Retrieved 2018-04-20.
- ^ Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, Huang M, Crawford J (October 2013). "The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials". Annals of Oncology. 24 (10): 2475–84. doi:10.1093/annonc/mdt226. PMC 3841419. PMID 23788754.
- ^ Willson ML, Burke L, Ferguson T, Ghersi D, Nowak AK, Wilcken N (September 2019). "Taxanes for adjuvant treatment of early breast cancer". The Cochrane Database of Systematic Reviews. 9 (9): CD004421. doi:10.1002/14651858.cd004421.pub3. PMC 6718224. PMID 31476253.
- ^ Bartlett JM, McConkey CC, Munro AF, Desmedt C, Dunn JA, Larsimont DP, O'Malley FP, Cameron DA, Earl HM, Poole CJ, Shepherd LE, Cardoso F, Jensen MB, Caldas C, Twelves CJ, Rea DW, Ejlertsen B, Di Leo A, Pritchard KI (May 2015). "Predicting Anthracycline Benefit: TOP2A and CEP17-Not Only but Also". Journal of Clinical Oncology. 33 (15): 1680–7. doi:10.1200/JCO.2013.54.7869. PMID 25897160.
- ^ "Discovery Of Method To Predict Which Breast Cancer Patients Should Be Treated With Anthracyclines". Medical News Today. 27 March 2010. Archived from the original on 28 January 2013.
- ^ PDQ Adult Treatment Editorial Board (2002). "Breast Cancer Treatment (Adult) (PDQ®): Patient Version". PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US). PMID 26389406.