Anaplastic oligodendroglioma
| Anaplastic oligodendroglioma | |
|---|---|
| Video of tumor growth simulation (anaplastic oligodendroglioma). Simulation was run for 2600 days since tumor onset (one original cell) with a detection level of 1 cell/mm2. | |
| Specialty | Neuro-oncology |
| Symptoms | Seizures[1] |
| Usual onset | peak years are age 45-50[1] |
| Duration | until cure or death |
| Causes | Generally unknown[1] |
| Risk factors | Generally unknown |
| Diagnostic method | Biopsy |
| Differential diagnosis | Other gliomas |
| Prevention | Not known |
| Treatment | Surgery, radiation, chemotherapy[1] |
| Medication | Temozolomide[1] |
| Prognosis | Generally fatal after 2-6 years[1] |
| Frequency | 0.07 to 0.18 per 100,000 person-years[1] |
Anaplastic oligodendroglioma is a neuroepithelial tumor which is believed to originate from oligodendrocytes, a cell type of the glia. In the World Health Organization (WHO) classification of brain tumors, anaplastic oligodendrogliomas are classified as grade III.[2] In the course of the disease, they can degenerate into WHO grade IV glioblastoma.[3] The vast majority of oligodendrogliomas occur sporadically, without a confirmed cause and without inheritance within a family.
Signs and symptoms
The clinical presentation of Anaplastic oligodendroglioma is as follow:[4]
- Seizures
- Headache
- Weakness
Pathogenesis
The (malignant) anaplastic oligodendroglioma belongs to the group of diffuse glioma and arises in the central nervous system (brain and spinal cord) from precursor stem cells of the oligodendrocytes. This tumor occurs primarily in middle adulthood with a frequency peak in the 4th and 5th decade of life.[3]
Diagnosis
The most important diagnostic procedure is magnetic resonance imaging (MRI).[3] Occasionally, outside of routine diagnostics, the metabolism in the tissue is shown using positron emission tomography (PET). The diagnosis is confirmed by a fine tissue examination following an operation. Anaplastic oligodendrogliomas often show a loss of genetic material. About 50 to 70%[5] of WHO grade III anaplastic oligodendrogliomas have combined allele losses on the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q). This change is mostly referred to as "1p / 19q Co Deletion". It can be seen as favorable for the patient and makes a response to radiation or chemotherapy more likely.[3] The designation of grade III oligodendroglioma (high grade) generally subsumes the previous diagnoses of anaplastic or malignant oligodendroglioma.[2]
-
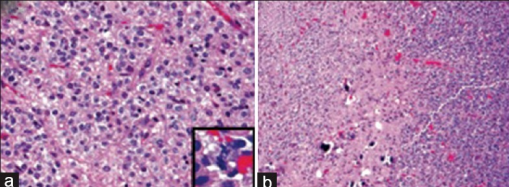
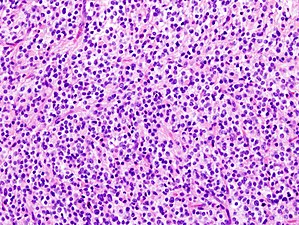
a)Anaplastic oligodendroglioma shows uniform cellularity, and perinuclear halos b)secondary features of oligodendroglioma include scattered microcalcifications
-
Histopathological image of anaplastic oligodendroglioma in cerebrum. Hematoxylin & eosin stain.
-
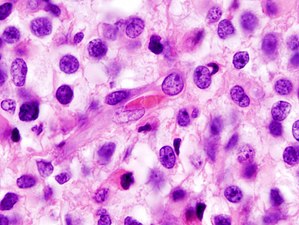
Zooming, note the irregular cell- and nucleus-shapes.
-
IDH1 R132H in anaplastic oligodendroglioma
Treatment
Surgery can help reduce symptoms caused by the tumor. As complete as possible removal of the tumor visible on the MRI is preferred, provided the location of the tumor allows this. Since typically the cells of an anaplastic oligodendroglioma have already migrated into the surrounding healthy brain tissue at the time of diagnosis, a complete surgical removal of all tumor cells is not possible. The "1p / 19q Codeletion" marker plays an increasingly important role in the selection of therapy and therapy combinations. Because this tumor is an "indolent condition" (a slowly progressive medical condition associated with little or no pain) and the potential morbidity associated with neurosurgery, chemotherapy and radiation therapy, most neurooncologists will initially pursue a course of watchful waiting and treat patients symptomatically. Symptomatic treatment often includes the use of anticonvulsants for seizures and steroids for brain swelling. For further treatment, radiation or chemotherapy with temozolomide or a chemotherapy with Procarbazine, CCNU and Vincristine (PCV) has been shown to be effective and was the most commonly used chemotherapy regimen used for treating anaplastic oligodendrogliomas.[3][6]
Prognosis
5–Year relative survival rate: Age 20–44, 76%. Age 45–54, 67%. Age 55–64, 45%.[7][8] Procarbazine, lomustine and vincristine have been used since May 1975. For 47 years, new therapeutic options have been regularly tested as part of therapy studies to improve the treatment of anaplastic oligodendroglioma.[9]
Research
As of 2021[update], a definitive cure is not possible with anaplastic oligodendrogliomas of WHO grade III. A retrospective study on 1054 patients with anaplastic oligodendroglioma, presented during the 2009 ASCO Annual Meeting, suggests that PCV therapy may be effective. Median time to progression for patients with 1p19q co-deletion was longer following PCV alone (7.6 years) than with temozolomide alone (3.3 years); median overall survival was also longer with PCV treatment versus temozolomide treatment (not reached, vs. 7.1 years).[10] A recent long-term study does affirm that radiation combined with adjuvant chemotherapy is significantly more efficacious for anaplastic oligodendroglioma patients with 1p 19q co-deleted tumors and has become the new standard of care.[11] It is possible that radiotherapy may prolong the overall time to progression for non-deleted tumors. If the tumor mass compresses adjacent brain structures, a neurosurgeon will typically remove as much of the tumor as he or she can without damaging other critical, healthy brain structures. Recent studies suggest that radiation does not improve overall survival (even when age, clinical data, histological grading, and type of surgery are considered).[12][13]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Anderson, Mark D.; Gilbert, Mark R. (April 14, 2013). "Treatment Recommendations for Anaplastic Oligodendrogliomas That Are Codeleted". Oncology. 27 (4). Archived from the original on 5 March 2021. Retrieved 4 January 2021.
- ↑ 2.0 2.1 Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (August 2007). "The 2007 WHO classification of tumours of the central nervous system". Acta Neuropathol. 114 (2): 97–109. doi:10.1007/s00401-007-0243-4. PMC 1929165. PMID 17618441.
- ↑ 3.0 3.1 3.2 3.3 3.4 Schweizerische Hirntumor Stiftung. "Anaplastisches Oligodendrogliom". www.swissbraintumorfoundation.com/ (in Deutsch). Archived from the original on 2020-02-02. Retrieved 2022-10-27.
- ↑ "Oligodendroglioma Diagnosis and Treatment - NCI". www.cancer.gov. 17 September 2018. Archived from the original on 9 April 2020. Retrieved 1 November 2022.
- ↑ administrador (5 February 2020). "Anaplastic oligodendroglioma, IDH-mutant & 1p/19q-codeleted". Archived from the original on 21 June 2022. Retrieved 27 October 2022.
- ↑ Engelhard HH, Stelea A, Mundt A (November 2003). "Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis". Surg Neurol. 60 (5): 443–56. doi:10.1016/s0090-3019(03)00167-8. PMID 14572971.
- ↑ "Survival Rates for Selected Adult Brain and Spinal Cord Tumors". American Cancer Society. Archived from the original on 30 December 2020. Retrieved 8 January 2021.
- ↑ Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18 Suppl 5:v1−v75. (November 7, 2017). "Survival Rates for Selected Adult Brain and Spinal Cord Tumors". Archived from the original on May 23, 2022. Retrieved October 27, 2022.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: uses authors parameter (link) - ↑ Gutin PH, Wilson CB, Kumar AR, Boldrey EB, Levin V, Powell M, Enot KJ (May 1975). "Phase II study of procarbazine, CCNU, and vincristine combination chemotherapy in the treatment of malignant brain tumors". Cancer. 35 (5): 1398–404. doi:10.1002/1097-0142(197505)35:5<1398::aid-cncr2820350524>3.0.co;2-c. PMID 1122488.
- ↑ Lassman, A. B. (20 May 2009). "Retrospective analysis of outcomes among more than 1,000 patients with newly diagnosed anaplastic oligodendroglial tumors". Journal of Clinical Oncology. 27 (15S): 2014. doi:10.1200/jco.2009.27.15_suppl.2014. ISSN 0732-183X.
- ↑ "New Standard of Care for Anaplastic Oligodendroglial Tumors with 1p/19q Codeletions – the ASCO Post". Archived from the original on 2021-10-20. Retrieved 2022-10-27.
- ↑ Sunyach MP, Jouvet A, Perol D, et al. (December 2007). "Role of exclusive chemotherapy as first line treatment in oligodendroglioma". J. Neurooncol. 85 (3): 319–28. doi:10.1007/s11060-007-9422-3. PMID 17568995. S2CID 27456056.
- ↑ Mohile NA, Forsyth P, Stewart D, et al. (September 2008). "A phase II study of intensified chemotherapy alone as initial treatment for newly diagnosed anaplastic oligodendroglioma: an interim analysis". J. Neurooncol. 89 (2): 187–93. doi:10.1007/s11060-008-9603-8. PMID 18458821. S2CID 30666549.
Further reading
- Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, A. S. Jakola, M. Platten, P. Roth, R. Rudà, S. Short, M. Smits, M. J. Taphoorn, A. von Deimling, M. Westphal, R. Soffietti, G. Reifenberger, W. Wick: EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. In: Nature reviews. Clinical oncology. Volume 18, Number 3, 03 2021, pp. 170-186, doi:10.1038/s41571-020-00447-z, PMID 33293629, PMC 7904519 (Review).
External links
- "Molekulare Diagnostik". Center for Neuropathology and Prion Research – Ludwig-Maximilians-Universität München. Archived from the original on 2021-10-22. Retrieved 2021-10-23.
- Pages with script errors
- CS1 Deutsch-language sources (de)
- CS1 errors: missing periodical
- CS1 maint: uses authors parameter
- Articles containing potentially dated statements from 2021
- Articles with invalid date parameter in template
- All articles containing potentially dated statements
- Brain tumor
- Cancer
- Types of cancer
- Oncology