Glioblastoma
| Glioblastoma | |
|---|---|
| Other names: Glioblastoma multiforme, grade IV astrocytoma | |
 | |
| Coronal MRI with contrast of a glioblastoma in a 15-year-old male | |
| Specialty | Oncology, neurosurgery |
| Symptoms | Initially nonspecific, headaches, personality changes, nausea, symptoms similar to a stroke[1] |
| Usual onset | ~ 64 years old[2][3] |
| Causes | Usually unclear[2] |
| Risk factors | Genetic disorders (neurofibromatosis, Li–Fraumeni syndrome), previous radiation therapy[2][3] |
| Diagnostic method | CT scan, MRI scan, tissue biopsy[1] |
| Prevention | Unknown[3] |
| Treatment | Surgery, chemotherapy, radiation[3] |
| Medication | Temozolomide, steroids[1][4] |
| Prognosis | Life expectancy ~ 14 months with treatment (5 year survival <7%)[2][5] |
| Frequency | 3 per 100,000 per year[3] |
Glioblastoma, also known as glioblastoma multiforme (GBM), is the most aggressive type of cancer that begins within the brain.[6] Initially, signs and symptoms of glioblastoma are nonspecific.[1] They may include headaches, personality changes, nausea and symptoms similar to those of a stroke.[1] Symptoms often worsen rapidly and may progress to unconsciousness.[2]
The cause of most cases of glioblastoma is not known.[2] Uncommon risk factors include genetic disorders, such as neurofibromatosis and Li–Fraumeni syndrome, and previous radiation therapy.[2][3] Glioblastomas represent 15% of all brain tumors.[1] They can either start from normal brain cells or develop from an existing low-grade astrocytoma.[7] The diagnosis typically is made by a combination of a CT scan, MRI scan and tissue biopsy.[1]
There is no known method of preventing the cancer.[3] Treatment usually involves surgery, after which chemotherapy and radiation therapy are used.[3] The medication temozolomide is frequently used as part of chemotherapy.[3][4][8] High-dose steroids may be used to help reduce swelling and decrease symptoms.[1] Greater surgical removal of the tumor is linked to longer survival.[9]
Despite maximum treatment, the cancer usually recurs.[3] The typical duration of survival following diagnosis is 12 to 15 months, with fewer than 3 to 7% of people surviving longer than five years.[2][5] Without treatment, survival is typically three months.[10] It is the most common cancer that begins within the brain and the second-most common brain tumor, after meningioma.[6][11] About 3 in 100,000 people develop the disease per year.[3] It most often begins around 64 years of age and occurs more commonly in males than females.[2][3] Immunotherapy is being studied as treatment for the cancer.[12]
Signs and symptoms
Common symptoms include seizures, headaches, nausea and vomiting, memory loss, changes to personality, mood or concentration, and localized neurological problems.[13] The kind of symptoms produced depends more on the location of the tumor than on its pathological properties. The tumor can start producing symptoms quickly, but occasionally is an asymptomatic condition until it reaches an enormous size.
Risk factors
The cause of most cases is unclear.[2] About 5% develop from another type brain tumor known as a low-grade astrocytoma.[13]
Genetics
Uncommon risk factors include genetic disorders such as neurofibromatosis, Li–Fraumeni syndrome, tuberous sclerosis, or Turcot syndrome.[13] Previous radiation therapy is also a risk.[2][3] For unknown reasons, it occurs more commonly in males.[14]
Environmental
Other associations include exposure to smoking, pesticides, and working in petroleum refining or rubber manufacturing.[13]
Glioblastoma has been associated with the viruses SV40,[15] HHV-6,[16][17] and cytomegalovirus.[18]
Other
Research has been done to see if consumption of cured meat is a risk factor. No risk had been confirmed as of 2013.[19] Similarly, exposure to radiation during medical imaging, formaldehyde, and residential electromagnetic fields, such as from cell phones and electrical wiring within homes, have been studied as risk factors. As of 2015, they had not been shown to cause GBM.[13][20][21] However, a meta-analysis published in 2007 found a correlation between the rate of GBMs and use of a cell phone for longer than 10 years, especially among those who always held the phone on one side of their heads.[13]
Pathogenesis
The cellular origin of glioblastoma is unknown. Because of the similarities in immunostaining of glial cells and glioblastoma, gliomas such as glioblastoma have long been assumed to originate from glial-type cells. More recent studies suggest that astrocytes, oligodendrocyte progenitor cells, and neural stem cells could all serve as the cell of origin.[22][23]
Glioblastomas are characterized by the presence of small areas of necrotizing tissue that are surrounded by anaplastic cells. This characteristic, as well as the presence of hyperplastic blood vessels, differentiates the tumor from grade 3 astrocytomas, which do not have these features.
GBMs usually form in the cerebral white matter, grow quickly, and can become very large before producing symptoms. Fewer than 10% form more slowly following degeneration of low-grade astrocytoma or anaplastic astrocytoma. These are called secondary GBMs and are more common in younger patients (mean age 45 versus 62 years).[24] The tumor may extend into the meninges or ventricular wall, leading to high protein content in the cerebrospinal fluid (CSF) (> 100 mg/dl), as well as an occasional pleocytosis of 10 to 100 cells, mostly lymphocytes. Malignant cells carried in the CSF may spread (rarely) to the spinal cord or cause meningeal gliomatosis. However, metastasis of GBM beyond the central nervous system is extremely unusual. About 50% of GBMs occupy more than one lobe of a hemisphere or are bilateral. Tumors of this type usually arise from the cerebrum and may exhibit the classic infiltration across the corpus callosum, producing a butterfly (bilateral) glioma.
Molecular alterations
Four subtypes of glioblastoma have been identified based on gene expression:[25]
- Classical: Around 97% of tumors in this subtype carry extra copies of the epidermal growth factor receptor (EGFR) gene, and most have higher than normal expression of EGFR, whereas the gene TP53 (p53), which is often mutated in glioblastoma, is rarely mutated in this subtype.[26] Loss of heterozygosity in chromosome 10 is also frequently seen in the classical subtype alongside chromosome 7 amplification.[27]
- The proneural subtype often has high rates of alterations in TP53 (p53), and in PDGFRA, the gene encoding a-type platelet-derived growth factor receptor, and in IDH1, the gene encoding isocitrate dehydrogenase-1.
- The mesenchymal subtype is characterized by high rates of mutations or other alterations in NF1, the gene encoding neurofibromin 1 and fewer alterations in the EGFR gene and less expression of EGFR than other types.[28]
- The neural subtype was typified by the expression of neuron markers such as NEFL, GABRA1, SYT1, and SLC12A5, while often presenting themselves as normal cells upon pathological assessment.[25][27]
Many other genetic alterations have been described in glioblastoma, and the majority of them are clustered in two pathways, the RB and the PI3K/AKT.[29] Glioblastomas have alterations in 68–78% and 88% of these pathways, respectively.[6]
Another important alteration is methylation of MGMT, a "suicide" DNA repair enzyme. Methylation impairs DNA transcription and expression of the MGMT gene. Since the MGMT enzyme can repair only one DNA alkylation due to its suicide repair mechanism, reverse capacity is low and methylation of the MGMT gene promoter greatly affects DNA-repair capacity.[30][31] Indeed, MGMT methylation is associated with an improved response to treatment with DNA-damaging chemotherapeutics, such as temozolomide.[32]
Cancer stem cells
Glioblastoma cells with properties similar to progenitor cells (glioblastoma cancer stem cells) have been found in glioblastomas. Their presence, coupled with the glioblastomas diffuse nature results in difficulty in removing them completely by surgery, and is therefore believed to be the possible cause behind resistance to conventional treatments, and the high recurrence rate.[33] Glioblastoma cancer stem cells share some resemblance with neural progenitor cells, both expressing the surface receptor CD133.[34] CD44 can also be used as a cancer stem cell marker in a subset of glioblastoma tumour cells.[35]
Metabolism
The IDH1 gene encodes for the enzyme isocitrate dehydrogenase 1 and is uncommonly mutated in glioblastoma (primary GBM: 5%, secondary GBM >80%).[31] By producing very high concentrations of the "oncometabolite" D-2-hydroxyglutarate and dysregulating the function of the wild-type IDH1 enzyme, it induces profound changes to the metabolism of IDH1-mutated glioblastoma, compared with IDH1 wild-type glioblastoma or healthy astrocytes. Among others, it increases the glioblastoma cells' dependence on glutamine or glutamate as an energy source.[36] IDH1-mutated glioblastomas are thought to have a very high demand for glutamate and use this amino acid and neurotransmitter as a chemotactic signal. Since healthy astrocytes excrete glutamate, IDH1-mutated glioblastoma cells do not favor dense tumor structures, but instead migrate, invade, and disperse into healthy parts of the brain where glutamate concentrations are higher. This may explain the invasive behavior of these IDH1-mutated glioblastoma.[37]
Ion channels
Furthermore, GBM exhibits numerous alterations in genes that encode for ion channels, including upregulation of gBK potassium channels and ClC-3 chloride channels. By upregulating these ion channels, glioblastoma tumor cells are hypothesized to facilitate increased ion movement over the cell membrane, thereby increasing H2O movement through osmosis, which aids glioblastoma cells in changing cellular volume very rapidly. This is helpful in their extremely aggressive invasive behavior because quick adaptations in cellular volume can facilitate movement through the sinuous extracellular matrix of the brain.[38]
MicroRNA
As of 2012, RNA interference, usually microRNA, was under investigation in tissue culture, pathology specimens, and preclinical animal models of glioblastoma.[39] Additionally, experimental observations suggest that microRNA-451 is a key regulator of LKB1/AMPK signaling in cultured glioma cells [40] and that miRNA clustering controls epigenetic pathways in the disease.[41]
Diagnosis

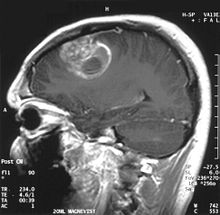


When viewed with MRI, glioblastomas often appear as ring-enhancing lesions. The appearance is not specific, however, as other lesions such as abscess, metastasis, tumefactive multiple sclerosis, and other entities may have a similar appearance.[42] Definitive diagnosis of a suspected GBM on CT or MRI requires a stereotactic biopsy or a craniotomy with tumor resection and pathologic confirmation. Because the tumor grade is based upon the most malignant portion of the tumor, biopsy or subtotal tumor resection can result in undergrading of the lesion. Imaging of tumor blood flow using perfusion MRI and measuring tumor metabolite concentration with MR spectroscopy may add diagnostic value to standard MRI in select cases by showing increased relative cerebral blood volume and increased choline peak, respectively, but pathology remains the gold standard for diagnosis and molecular characterization.
Distinguishing primary glioblastoma from secondary glioblastoma is important. These tumors occur spontaneously (de novo) or have progressed from a lower-grade glioma, respectively.[6] Primary glioblastomas have a worse prognosis and different tumor biology, and may have a different response to therapy, which makes this a critical evaluation to determine patient prognosis and therapy.[30] Over 80% of secondary glioblastomas carry a mutation in IDH1, whereas this mutation is rare in primary glioblastoma (5–10%). Thus, IDH1 mutations are a useful tool to distinguish primary and secondary glioblastomas, since histopathologically they are very similar and the distinction without molecular biomarkers is unreliable.[31]
Prevention
There are no known methods to prevent glioblastoma.[3]
Treatment
Treating glioblastoma is very difficult due to several complicating factors:[43]
- The tumor cells are very resistant to conventional therapies.
- The brain is susceptible to damage from conventional therapy.
- The brain has a very limited capacity to repair itself.
- Many drugs cannot cross the blood–brain barrier to act on the tumor.
Treatment of primary brain tumors consists of palliative (symptomatic) care and therapies intended to improve survival.
Symptomatic therapy
Supportive treatment focuses on relieving symptoms and improving the patient’s neurologic function. The primary supportive agents are anticonvulsants and corticosteroids.
- Historically, around 90% of patients with glioblastoma underwent anticonvulsant treatment, although only an estimated 40% of patients required this treatment. Recently, neurosurgeons have been recommended that anticonvulsants not be administered prophylactically, and should wait until a seizure occurs before prescribing this medication.[44] Those receiving phenytoin concurrent with radiation may have serious skin reactions such as erythema multiforme and Stevens–Johnson syndrome.
- Corticosteroids, usually dexamethasone, can reduce peritumoral edema (through rearrangement of the blood–brain barrier), diminishing mass effect and lowering intracranial pressure, with a decrease in headache or drowsiness.
Surgery
Surgery is the first stage of treatment of glioblastoma. An average GBM tumor contains 1011 cells, which is on average reduced to 109 cells after surgery (a reduction of 99%). Benefits of surgery include resection for a pathological diagnosis, alleviation of symptoms related to mass effect, and potentially removing disease before secondary resistance to radiotherapy and chemotherapy occurs.
The greater the extent of tumor removal, the better. In retrospective analyses, removal of 98% or more of the tumor has been associated with a significantly longer healthier time than if less than 98% of the tumor is removed.[45] The chances of near-complete initial removal of the tumor may be increased if the surgery is guided by a fluorescent dye known as 5-aminolevulinic acid.[46] GBM cells are widely infiltrative through the brain at diagnosis, so despite a "total resection" of all obvious tumor, most people with GBM later develop recurrent tumors either near the original site or at more distant locations within the brain. Other modalities, typically radiation and chemotherapy, are used after surgery in an effort to suppress and slow recurrent disease.
Radiotherapy
Subsequent to surgery, radiotherapy becomes the mainstay of treatment for people with glioblastoma. It is typically performed along with giving temozolomide.[8] A pivotal clinical trial carried out in the early 1970s showed that among 303 GBM patients randomized to radiation or nonradiation therapy, those who received radiation had a median survival more than double those who did not.[47] Subsequent clinical research has attempted to build on the backbone of surgery followed by radiation. On average, radiotherapy after surgery can reduce the tumor size to 107 cells. Whole-brain radiotherapy does not improve when compared to the more precise and targeted three-dimensional conformal radiotherapy.[48] A total radiation dose of 60–65 Gy has been found to be optimal for treatment.[49]
GBM tumors are well known to contain zones of tissue exhibiting hypoxia, which are highly resistant to radiotherapy. Various approaches to chemotherapy radiosensitizers have been pursued with limited success as of 2016[update]. As of 2010[update], newer research approaches included preclinical and clinical investigations into the use of an oxygen diffusion-enhancing compound such as trans sodium crocetinate as radiosensitizers,[50] and as of 2015[update] a clinical trial was underway.[51] Boron neutron capture therapy has been tested as an alternative treatment for glioblastoma, but is not in common use.
Chemotherapy
Most studies show no benefit from the addition of chemotherapy. However, a large clinical trial of 575 participants randomized to standard radiation versus radiation plus temozolomide chemotherapy showed that the group receiving temozolomide survived a median of 14.6 months as opposed to 12.1 months for the group receiving radiation alone.[8][52] This treatment regimen is now standard for most cases of glioblastoma where the person is not enrolled in a clinical trial.[53][54] Temozolomide seems to work by sensitizing the tumor cells to radiation, and appears more effective for tumors with MGMT promoter methylation.[55] High doses of temozolomide in high-grade gliomas yield low toxicity, but the results are comparable to the standard doses.[56] Antiangiogenic therapy with medications such as bevacizumab control symptoms, but do not appear to affect overall survival in those with glioblastoma.[57] The overall benefit of anti-angiogneic therapies as of 2019 is unclear.[57] In elderly people with newly diagnosed glioblastoma who are reasonably fit, concurrent and adjuvant chemoradiotherapy gives the best overall survival but is associated with a greater risk of haematological adverse events than radiotherapy alone.[58]
Other procedures
Alternating electric field therapy is an FDA-approved therapy for newly diagnosed[59] and recurrent glioblastoma.[60] In 2015, initial results from a phase-three randomized clinical trial of alternating electric field therapy plus temozolomide in newly diagnosed glioblastoma reported a three-month improvement in progression-free survival, and a five-month improvement in overall survival compared to temozolomide therapy alone,[61][62] representing the first large trial in a decade to show a survival improvement in this setting.[62] Despite these results, the efficacy of this approach remains controversial among medical experts.[63]
Prognosis
The most common length of survival following diagnosis is 12 to 15 months, with fewer than 3 to 7% of people surviving longer than five years.[2][5] In the United States between 2012 and 2016 five year survival was 6.8%.[5] Without treatment, survival is typically 3 months.[10]
Increasing age (> 60 years) carries a worse prognostic risk. Death is usually due to widespread tumor infiltration with cerebral edema and increased intracranial pressure.[64]
A good initial Karnofsky performance score (KPS) and MGMT methylation are associated with longer survival.[64] A DNA test can be conducted on glioblastomas to determine whether or not the promoter of the MGMT gene is methylated. Patients with a methylated MGMT promoter have longer survival than those with an unmethylated MGMT promoter, due in part to increased sensitivity to temozolomide.[65] This DNA characteristic is intrinsic to the patient and currently cannot be altered externally. Another positive prognostic marker for glioblastoma patients is mutation of the IDH1 gene,[6] which can be tested by DNA-based methods or by immunohistochemistry using an antibody against the most common mutation, namely IDH1-R132H.[66]
More prognostic power can be obtained by combining the mutational status of IDH1 and the methylation status of MGMT into a two-gene predictor. Patients with both IDH1 mutations and MGMT methylation have the longest survival, patients with an IDH1 mutation or MGMT methylation an intermediate survival, and patients without either genetic event have the shortest survival.[30]
Long-term benefits have also been associated with those patients who receive surgery, radiotherapy, and temozolomide chemotherapy.[64] However, much remains unknown about why some patients survive longer with glioblastoma. Age under 50 is linked to longer survival in GBM, as is 98%+ resection and use of temozolomide chemotherapy and better KPSs. A recent study confirms that younger age is associated with a much better prognosis, with a small fraction of patients under 40 years of age achieving a population-based cure. Cure is thought to occur when a person's risk of death returns to that of the normal population, and in GBM, this is thought to occur after 10 years.[67]
UCLA Neuro-oncology publishes real-time survival data for patients with this diagnosis.[68] They are the only institution in the United States that shows how their patients are performing. They also show a listing of chemotherapy agents used to treat GBM tumors. Despite a poor prognosis, a small number of survivors have been GBM free for more than 10 years.
According to a 2003 study, GBM prognosis can be divided into three subgroups dependent on KPS, the age of the patient, and treatment.[69]
| Recursive partitioning analysis (RPA) class |
Definition | Historical Median Survival Time | Historical 1-Year Survival | Historical 3-Year Survival | Historical 5-Year Survival |
|---|---|---|---|---|---|
| III | Age < 50, KPS ≥ 90 | 17.1 months | 70% | 20% | 14% |
| IV | Age < 50, KPS < 90 | 11.2 months | 46% | 7% | 4% |
| Age ≥ 50, KPS ≥ 70, surgical removal with good neurologic function | |||||
| V + VI | Age ≥ 50, KPS ≥ 70, surgical removal with poor neurologic function | 7.5 months | 28% | 1% | 0% |
| Age ≥ 50, KPS ≥ 70, no surgical removal | |||||
| Age ≥ 50, KPS < 70 |
Epidemiology
About three per 100,000 people develop the disease a year,[3] although regional frequency may be much higher.[70] The frequency in England has doubled between 1995 and 2015.[71]
It is the second-most common central nervous system cancer after meningioma.[11] It occurs more commonly in males than females.[2][3] Although it most often begins around 64 years of age,[2][3] in 2014, the broad category of brain cancers was second only to leukemia in people in the United States under 20 years of age.[72]
History
The term glioblastoma multiforme was introduced in 1926 by Percival Bailey and Harvey Cushing, based on the idea that the tumor originates from primitive precursors of glial cells (glioblasts), and the highly variable appearance due to the presence of necrosis, hemorrhage, and cysts (multiform).[73]
Research
Gene therapy
Gene therapy has been explored as a method to treat glioblastoma, and while animal models and early-phase clinical trials have been successful, as of 2017, all gene-therapy drugs that had been tested in phase III clinical trials for glioblastoma had failed.[74][75][76]
Intranasal drug delivery
Direct nose-to-brain drug delivery is being explored as a means to achieve higher, and hopefully more effective, drug concentrations in the brain.[77][78] A clinical phase I/II study with glioblastoma patients in Brazil investigated the natural compound perillyl alcohol for intranasal delivery as an aerosol. The results were encouraging[77][79][80] and, as of 2016, a similar trial has been initiated in the United States.[81]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Young RM, Jamshidi A, Davis G, Sherman JH (June 2015). "Current trends in the surgical management and treatment of adult glioblastoma". Annals of Translational Medicine. 3 (9): 121. doi:10.3978/j.issn.2305-5839.2015.05.10. PMC 4481356. PMID 26207249.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "Chapter 5.16". World Cancer Report 2014. World Health Organization. 2014. ISBN 978-9283204299.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 Gallego O (August 2015). "Nonsurgical treatment of recurrent glioblastoma". Current Oncology. 22 (4): e273-81. doi:10.3747/co.22.2436. PMC 4530825. PMID 26300678.
- ↑ 4.0 4.1 Hart MG, Garside R, Rogers G, Stein K, Grant R (April 2013). "Temozolomide for high grade glioma". The Cochrane Database of Systematic Reviews. 4 (4): CD007415. doi:10.1002/14651858.CD007415.pub2. PMC 6457743. PMID 23633341.
- ↑ 5.0 5.1 5.2 5.3 Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (November 2019). "CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016". Neuro-Oncology. 21 (Supplement_5): v1–v100. doi:10.1093/neuonc/noz150. PMC 6823730. PMID 31675094.
- ↑ 6.0 6.1 6.2 6.3 6.4 Bleeker FE, Molenaar RJ, Leenstra S (May 2012). "Recent advances in the molecular understanding of glioblastoma". Journal of Neuro-Oncology. 108 (1): 11–27. doi:10.1007/s11060-011-0793-0. PMC 3337398. PMID 22270850.
- ↑ "Chapter 3.8". World Cancer Report 2014. World Health Organization. 2014. ISBN 978-9283204299.
- ↑ 8.0 8.1 8.2 Khosla D (February 2016). "Concurrent therapy to enhance radiotherapeutic outcomes in glioblastoma". Annals of Translational Medicine. 4 (3): 54. doi:10.3978/j.issn.2305-5839.2016.01.25. PMC 4740000. PMID 26904576.
- ↑ Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010). "Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma". Ca. 60 (3): 166–93. doi:10.3322/caac.20069. PMC 2888474. PMID 20445000.
- ↑ 10.0 10.1 Schapira, Anthony H.V. (2007). Neurology and clinical neuroscience. Philadelphia: Mosby Elsevier. p. 1336. ISBN 9780323070539. Archived from the original on 2017-07-29.
- ↑ 11.0 11.1 McNeill KA (November 2016). "Epidemiology of Brain Tumors". Neurologic Clinics. 34 (4): 981–998. doi:10.1016/j.ncl.2016.06.014. PMID 27720005.
- ↑ "With Immunotherapy, Glimmers of Progress against Glioblastoma". National Cancer Institute. 9 December 2015. Archived from the original on 24 December 2015. Retrieved 23 December 2015.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Alifieris C, Trafalis DT (August 2015). "Glioblastoma multiforme: Pathogenesis and treatment". Pharmacology & Therapeutics. 152: 63–82. doi:10.1016/j.pharmthera.2015.05.005. PMID 25944528.
- ↑ Ohgaki H, Kleihues P (June 2005). "Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas". Journal of Neuropathology and Experimental Neurology. 64 (6): 479–89. doi:10.1093/jnen/64.6.479. PMID 15977639.
- ↑ Vilchez RA, Kozinetz CA, Arrington AS, Madden CR, Butel JS (June 2003). "Simian virus 40 in human cancers". The American Journal of Medicine. 114 (8): 675–84. doi:10.1016/S0002-9343(03)00087-1. PMID 12798456.
- ↑ Crawford JR, Santi MR, Thorarinsdottir HK, Cornelison R, Rushing EJ, Zhang H, et al. (September 2009). "Detection of human herpesvirus-6 variants in pediatric brain tumors: association of viral antigen in low grade gliomas". Journal of Clinical Virology. 46 (1): 37–42. doi:10.1016/j.jcv.2009.05.011. PMC 2749001. PMID 19505845.
- ↑ Chi J, Gu B, Zhang C, Peng G, Zhou F, Chen Y, et al. (November 2012). "Human herpesvirus 6 latent infection in patients with glioma". The Journal of Infectious Diseases. 206 (9): 1394–8. doi:10.1093/infdis/jis513. PMID 22962688.
- ↑ McFaline-Figueroa JR, Wen PY (February 2017). "The Viral Connection to Glioblastoma". Current Infectious Disease Reports. 19 (2): 5. doi:10.1007/s11908-017-0563-z. PMID 28233187.
- ↑ Huncharek M, Kupelnick B, Wheeler L (2003). "Dietary cured meat and the risk of adult glioma: a meta-analysis of nine observational studies". Journal of Environmental Pathology, Toxicology and Oncology. 22 (2): 129–37. doi:10.1615/JEnvPathToxOncol.v22.i2.60. PMID 14533876.
- ↑ Kan P, Simonsen SE, Lyon JL, Kestle JR (January 2008). "Cellular phone use and brain tumor: a meta-analysis". Journal of Neuro-Oncology. 86 (1): 71–8. doi:10.1007/s11060-007-9432-1. PMID 17619826.
- ↑ Hardell L, Carlberg M, Hansson Mild K (August 2009). "Epidemiological evidence for an association between use of wireless phones and tumor diseases". Pathophysiology. 16 (2–3): 113–22. doi:10.1016/j.pathophys.2009.01.003. PMID 19268551.
- ↑ Zong H, Verhaak RG, Canoll P (May 2012). "The cellular origin for malignant glioma and prospects for clinical advancements". Expert Review of Molecular Diagnostics. 12 (4): 383–94. doi:10.1586/erm.12.30. PMC 3368274. PMID 22616703.
- ↑ Zong H, Parada LF, Baker SJ (January 2015). "Cell of origin for malignant gliomas and its implication in therapeutic development". Cold Spring Harbor Perspectives in Biology. 7 (5): a020610. doi:10.1101/cshperspect.a020610. PMC 4448618. PMID 25635044.
- ↑ Ohgaki H, Kleihues P (December 2009). "Genetic alterations and signaling pathways in the evolution of gliomas". Cancer Science. 100 (12): 2235–41. doi:10.1111/j.1349-7006.2009.01308.x. PMID 19737147.
- ↑ 25.0 25.1 Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. (January 2010). "Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1". Cancer Cell. 17 (1): 98–110. doi:10.1016/j.ccr.2009.12.020. PMC 2818769. PMID 20129251.
- ↑ Hayden EC (January 2010). "Genomics boosts brain-cancer work". Nature. 463 (7279): 278. doi:10.1038/463278a. PMID 20090720.
- ↑ 27.0 27.1 Sasmita AO, Wong YP, Ling AP (February 2018). "Biomarkers and therapeutic advances in glioblastoma multiforme". Asia-Pacific Journal of Clinical Oncology. 14 (1): 40–51. doi:10.1111/ajco.12756. PMID 28840962.

- ↑ Kuehn BM (March 2010). "Genomics illuminates a deadly brain cancer". JAMA. 303 (10): 925–7. doi:10.1001/jama.2010.236. PMID 20215599.
- ↑ Bleeker FE, Lamba S, Zanon C, Molenaar RJ, Hulsebos TJ, Troost D, et al. (September 2014). "Mutational profiling of kinases in glioblastoma". BMC Cancer. 14 (1): 718. doi:10.1186/1471-2407-14-718. PMC 4192443. PMID 25256166.
- ↑ 30.0 30.1 30.2 Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH, et al. (September 2014). "The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone". Neuro-Oncology. 16 (9): 1263–73. doi:10.1093/neuonc/nou005. PMC 4136888. PMID 24510240.
- ↑ 31.0 31.1 31.2 Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE (December 2014). "The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1846 (2): 326–41. doi:10.1016/j.bbcan.2014.05.004. PMID 24880135.
- ↑ Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. (March 2005). "MGMT gene silencing and benefit from temozolomide in glioblastoma" (PDF). The New England Journal of Medicine. 352 (10): 997–1003. doi:10.1056/NEJMoa043331. PMID 15758010. Archived (PDF) from the original on 2018-07-19. Retrieved 2019-08-16.
- ↑ Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, et al. (June 2008). "Stem cell-related "self-renewal" signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma". Journal of Clinical Oncology. 26 (18): 3015–24. doi:10.1200/JCO.2007.15.7164. PMID 18565887.
- ↑ Gilbertson RJ, Rich JN (October 2007). "Making a tumour's bed: glioblastoma stem cells and the vascular niche". Nature Reviews. Cancer. 7 (10): 733–6. doi:10.1038/nrc2246. PMID 17882276.
- ↑ Brown DV, Stylli SS, Kaye AH, Mantamadiotis T (2019). "Multilayered Heterogeneity of Glioblastoma Stem Cells: Biological and Clinical Significance". Advances in Experimental Medicine and Biology. 1139: 1–21. doi:10.1007/978-3-030-14366-4_1. ISBN 978-3-030-14365-7. PMID 31134492.
- ↑ van Lith SA, Navis AC, Verrijp K, Niclou SP, Bjerkvig R, Wesseling P, et al. (August 2014). "Glutamate as chemotactic fuel for diffuse glioma cells: are they glutamate suckers?". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1846 (1): 66–74. doi:10.1016/j.bbcan.2014.04.004. PMID 24747768.
- ↑ van Lith SA, Molenaar R, van Noorden CJ, Leenders WP (December 2014). "Tumor cells in search for glutamate: an alternative explanation for increased invasiveness of IDH1 mutant gliomas". Neuro-Oncology. 16 (12): 1669–70. doi:10.1093/neuonc/nou152. PMC 4232089. PMID 25074540.
- ↑ Molenaar RJ (2011). "Ion channels in glioblastoma". ISRN Neurology. 2011: 590249. doi:10.5402/2011/590249. PMC 3263536. PMID 22389824.
- ↑ Møller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M (February 2013). "A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion". Molecular Neurobiology. 47 (1): 131–44. doi:10.1007/s12035-012-8349-7. PMC 3538124. PMID 23054677.
- ↑ Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, et al. (March 2010). "MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells". Molecular Cell. 37 (5): 620–32. doi:10.1016/j.molcel.2010.02.018. PMC 3125113. PMID 20227367.
- ↑ Bhaskaran V, Nowicki MO, Idriss M, Jimenez MA, Lugli G, Hayes JL, et al. (January 2019). "The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma". Nature Communications. 10 (1): 442. Bibcode:2019NatCo..10..442B. doi:10.1038/s41467-019-08390-z. PMC 6347618. PMID 30683859.
- ↑ Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW (2007). "Patterns of contrast enhancement in the brain and meninges". Radiographics. 27 (2): 525–51. doi:10.1148/rg.272065155. PMID 17374867.
- ↑ Lawson HC, Sampath P, Bohan E, Park MC, Hussain N, Olivi A, et al. (May 2007). "Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience". Journal of Neuro-Oncology. 83 (1): 61–70. doi:10.1007/s11060-006-9303-1. PMC 4086528. PMID 17171441.
- ↑ Stevens GH (July 2006). "Antiepileptic therapy in patients with central nervous system malignancies". Current Neurology and Neuroscience Reports. 6 (4): 311–8. doi:10.1007/s11910-006-0024-9. PMID 16822352.
- ↑ Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. (August 2001). "A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival". Journal of Neurosurgery. 95 (2): 190–8. doi:10.3171/jns.2001.95.2.0190. PMID 11780887.
- ↑ Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (May 2006). "Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial". The Lancet. Oncology. 7 (5): 392–401. doi:10.1016/S1470-2045(06)70665-9. PMID 16648043.
- ↑ Walker MD, Alexander E, Hunt WE, MacCarty CS, Mahaley MS, Mealey J, et al. (September 1978). "Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial". Journal of Neurosurgery. 49 (3): 333–43. doi:10.3171/jns.1978.49.3.0333. PMID 355604.
- ↑ Showalter TN, Andrel J, Andrews DW, Curran WJ, Daskalakis C, Werner-Wasik M (November 2007). "Multifocal glioblastoma multiforme: prognostic factors and patterns of progression". International Journal of Radiation Oncology, Biology, Physics. 69 (3): 820–4. doi:10.1016/j.ijrobp.2007.03.045. PMID 17499453.
- ↑ Fulton DS, Urtasun RC, Scott-Brown I, Johnson ES, Mielke B, Curry B, et al. (September 1992). "Increasing radiation dose intensity using hyperfractionation in patients with malignant glioma. Final report of a prospective phase I-II dose response study". Journal of Neuro-Oncology. 14 (1): 63–72. doi:10.1007/BF00170946. PMID 1335044.
- ↑ Sheehan JP, Shaffrey ME, Gupta B, Larner J, Rich JN, Park DM (October 2010). "Improving the radiosensitivity of radioresistant and hypoxic glioblastoma". Future Oncology. 6 (10): 1591–601. doi:10.2217/fon.10.123. PMID 21062158.
- ↑ Clinical trial number NCT01465347 for "Safety and Efficacy Study of Trans Sodium Crocetinate (TSC) With Concomitant Radiation Therapy and Temozolomide in Newly Diagnosed Glioblastoma (GBM)" at ClinicalTrials.gov, accessed 2016-02-01
- ↑ Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. (European Organisation for Research Treatment of Cancer Brain Tumor Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group) (March 2005). "Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma". The New England Journal of Medicine. 352 (10): 987–96. doi:10.1056/NEJMoa043330. PMID 15758009. Archived from the original on 2019-10-14. Retrieved 2018-10-09.
- ↑ Mason WP, Mirimanoff RO, Stupp R (2006). Radiotherapy with Concurrent and Adjuvant Temozolomide: A New Standard of Care for Glioblastoma Multiforme. Progress in Neurotherapeutics and Neuropsychopharmacology. Vol. 1. pp. 37–52. doi:10.1017/S1748232105000054. ISBN 978-0-521-86253-0. Archived from the original on 2015-03-17.
- ↑ "Temozolomide Plus Radiation Helps Brain Cancer – National Cancer Institute". Archived from the original on August 15, 2007. Retrieved 2007-09-15.
- ↑ Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE (March 2007). "Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma". Journal of Neuro-Oncology. 82 (1): 81–3. doi:10.1007/s11060-006-9241-y. PMID 16944309.
- ↑ Dall'oglio S, D'Amico A, Pioli F, Gabbani M, Pasini F, Passarin MG, et al. (December 2008). "Dose-intensity temozolomide after concurrent chemoradiotherapy in operated high-grade gliomas". Journal of Neuro-Oncology. 90 (3): 315–9. doi:10.1007/s11060-008-9663-9. PMID 18688571.
- ↑ 57.0 57.1 Ameratunga M, Pavlakis N, Wheeler H, Grant R, Simes J, Khasraw M (November 2018). "Anti-angiogenic therapy for high-grade glioma". The Cochrane Database of Systematic Reviews. 11: CD008218. doi:10.1002/14651858.CD008218.pub4. PMC 6516839. PMID 30480778.
The use of anti-angiogenic therapy does not significantly improve overall survival in newly diagnosed people with glioblastoma. Thus, there is insufficient evidence to support the use of anti-angiogenic therapy for people with newly diagnosed glioblastoma at this time.
- ↑ Hanna C, Lawrie TA, Rogozińska E, Kernohan A, Jefferies S, Bulbeck H, et al. (March 2020). "Treatment of newly diagnosed glioblastoma in the elderly: a network meta-analysis". The Cochrane Database of Systematic Reviews. 3: CD013261. doi:10.1002/14651858.cd013261.pub2. PMC 7086476. PMID 32202316.
- ↑ "FDA approves expanded indication for medical device to treat a form of brain cancer". Archived from the original on 23 March 2016. Retrieved 19 March 2016.
- ↑ "FDA approval letter – NovoTTF-100A System" (PDF). www.fda.gov. Archived (PDF) from the original on 22 September 2015. Retrieved 26 December 2014.
- ↑ Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. (December 2015). "Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial". JAMA. 314 (23): 2535–43. doi:10.1001/jama.2015.16669. PMID 26670971.
- ↑ 62.0 62.1 Sampson JH (December 2015). "Alternating Electric Fields for the Treatment of Glioblastoma". JAMA. 314 (23): 2511–3. doi:10.1001/jama.2015.16701. PMID 26670969.
- ↑ Wick W (March 2016). "TTFields: where does all the skepticism come from?". Neuro-Oncology. 18 (3): 303–5. doi:10.1093/neuonc/now012. PMC 4767251. PMID 26917587.
- ↑ 64.0 64.1 64.2 Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. (October 2007). "Long-term survival with glioblastoma multiforme". Brain. 130 (Pt 10): 2596–606. doi:10.1093/brain/awm204. PMID 17785346.
- ↑ Martinez R, Schackert G, Yaya-Tur R, Rojas-Marcos I, Herman JG, Esteller M (May 2007). "Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme". Journal of Neuro-Oncology. 83 (1): 91–3. doi:10.1007/s11060-006-9292-0. PMID 17164975.
- ↑ Preusser M, Wöhrer A, Stary S, Höftberger R, Streubel B, Hainfellner JA (August 2011). "Value and limitations of immunohistochemistry and gene sequencing for detection of the IDH1-R132H mutation in diffuse glioma biopsy specimens". Journal of Neuropathology and Experimental Neurology. 70 (8): 715–23. doi:10.1097/NEN.0b013e31822713f0. PMID 21760534.
- ↑ Smoll NR, Schaller K, Gautschi OP (2012). "The cure fraction of glioblastoma multiforme". Neuroepidemiology. 39 (1): 63–9. doi:10.1159/000339319. PMID 22776797.
- ↑ University of California, Los Angeles Neuro-Oncology : How Our Patients Perform : Glioblastoma Multiforme [GBM] Archived 2012-06-09 at the Wayback Machine. Neurooncology.ucla.edu. Retrieved on 2010-10-19.
- ↑ Shaw EG, Seiferheld W, Scott C, Coughlin C, Leibel S, Curran W, Mehta M (2003). "Reexamining the radiation therapy oncology group (RTOG) recursive partitioning analysis (RPA) for glioblastoma multiforme (GBM) patients". International Journal of Radiation Oncology*Biology*Physics. 57 (2): S135–36. doi:10.1016/S0360-3016(03)00843-5.
- ↑ Xu H, Chen J, Xu H, Qin Z (2017). "Geographic Variations in the Incidence of Glioblastoma and Prognostic Factors Predictive of Overall Survival in US Adults from 2004-2013". Frontiers in Aging Neuroscience. 9: 352. doi:10.3389/fnagi.2017.00352. PMC 5681990. PMID 29163134.
- ↑ Philips A, Henshaw DL, Lamburn G, O'Carroll MJ (2018). "Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995-2015 Suggests an Adverse Environmental or Lifestyle Factor". Journal of Environmental and Public Health. 2018: 7910754. doi:10.1155/2018/7910754. PMC 6035820. PMID 30034480.
- ↑ Siegel DA, Li J, Henley SJ, Wilson RJ, Lunsford NB, Tai E, Van Dyne EA (June 2018). "Geographic Variation in Pediatric Cancer Incidence - United States, 2003-2014". MMWR. Morbidity and Mortality Weekly Report. 67 (25): 707–713. doi:10.15585/mmwr.mm6725a2. PMC 6023185. PMID 29953430.
- ↑ Bailey & Cushing: Tumors of the Glioma Group. JB Lippincott, Philadelphia, 1926.[page needed]
- ↑ Rajesh Y, Pal I, Banik P, Chakraborty S, Borkar SA, Dey G, et al. (May 2017). "Insights into molecular therapy of glioma: current challenges and next generation blueprint". Acta Pharmacologica Sinica. 38 (5): 591–613. doi:10.1038/aps.2016.167. PMC 5457688. PMID 28317871.
- ↑ Tobias A, Ahmed A, Moon KS, Lesniak MS (February 2013). "The art of gene therapy for glioma: a review of the challenging road to the bedside". Journal of Neurology, Neurosurgery, and Psychiatry. 84 (2): 213–22. doi:10.1136/jnnp-2012-302946. PMC 3543505. PMID 22993449.
- ↑ Fulci G, Chiocca EA (February 2007). "The status of gene therapy for brain tumors". Expert Opinion on Biological Therapy. 7 (2): 197–208. doi:10.1517/14712598.7.2.197. PMC 2819130. PMID 17250458.
- ↑ 77.0 77.1 van Woensel M, Wauthoz N, Rosière R, Amighi K, Mathieu V, Lefranc F, et al. (August 2013). "Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM?". Cancers. 5 (3): 1020–48. doi:10.3390/cancers5031020. PMC 3795377. PMID 24202332.
- ↑ Pardeshi CV, Belgamwar VS (July 2013). "Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting". Expert Opinion on Drug Delivery. 10 (7): 957–72. doi:10.1517/17425247.2013.790887. PMID 23586809.
- ↑ Peterson A, Bansal A, Hofman F, Chen TC, Zada G (February 2014). "A systematic review of inhaled intranasal therapy for central nervous system neoplasms: an emerging therapeutic option". Journal of Neuro-Oncology. 116 (3): 437–46. doi:10.1007/s11060-013-1346-5. PMID 24398618.
- ↑ Chen TC, Fonseca CO, Schönthal AH (2015). "Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy". American Journal of Cancer Research. 5 (5): 1580–93. PMC 4497427. PMID 26175929.
- ↑ Clinical trial number NCT02704858 for "Safety and Efficacy Study in Recurrent Grade IV Glioma" at ClinicalTrials.gov
External links
| Classification | |
|---|---|
| External resources |
- Information about Glioblastoma Multiforme (GBM) from the American Brain Tumor Association
- AFIP Course Syllabus – Astrocytoma WHO Grading Lecture Handout
- Pages with script errors
- Webarchive template wayback links
- Wikipedia articles needing page number citations from August 2013
- Articles with invalid date parameter in template
- Articles containing potentially dated statements from 2016
- All articles containing potentially dated statements
- Articles containing potentially dated statements from 2010
- Articles containing potentially dated statements from 2015
- Brain tumor
- RTT
- Cancer
- Types of cancer
- Oncology
- Aging-associated diseases