User talk:QuackGuru/Sand A
{{Db-u1|No longer needed.}} https://commons.wikimedia.org/wiki/Special:Watchlist
https://commons.wikimedia.org/w/index.php?title=User:QuackGuru/2024Requests&action=edit&redlink=1
https://commons.wikimedia.org/w/index.php?title=User:QuackGuru/2024Workshop&action=edit&redlink=1
Image being zoomed in
https://commons.wikimedia.org/wiki/File:The_gout_james_gillray(KenBurns).webm A gif of a picture zooming in would work better.
Requests
Discussion on requests
I need your professional expertise for creating a map. All the specific instructions are below. I'm trying to complete all my requests this year. I hope you have some free time to complete just one request. Thanks.
Vaping lung disease outbreak map
Upload a new file under the name "File:2019–2020 vaping lung disease outbreak - fatalities.svg"
Various states need to be updated in the new map under a new file name.
See: "Alabama, California (4)" For example, Alabama (AL) needs to be changed to orange. CA is already orange. No change needed for California (CA).
See: "Connecticut (CT), Delaware (DE), District of Columbia (DC), Florida (FL) (2)"
See: "Georgia (GA) (6)"
See "Illinois (IL) (5)"
See "Indiana (IN) (6)"
See "Kansas (KS) (2)"
See "Kentucky (KY), Louisiana (LA) (2)"
See "Massachusetts (MA) (5)"
See "Michigan (MI) (3)"
See "Minnesota (MN) (3)"
See "Mississippi (MS), Missouri (MO) (2)"
See "Montana (MT), Nebraska (NE), New Jersey (NJ), New York (NY) (4)"
See "Oregon (OR) (2)"
See "Pennsylvania (PA), Rhode Island (RI), South Carolina (SC), Tennessee (TN) (2)"
See "Texas (TX) (4)"
See "Utah (UT), Virginia (VA) and Washington (WA) (2)"
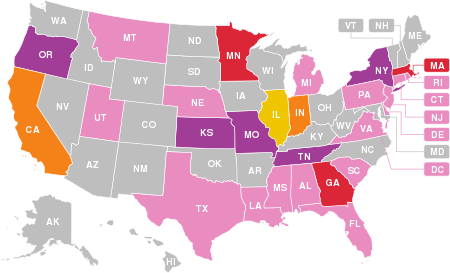
69 deaths associated with the use of vaping products have been confirmed in the US, as of February 18, 2020[7] - this map shows states with confirmed fatalities.
- Indicates at least one death linked to a vaping product
- Indicates at least two deaths linked to a vaping product
- Indicates at least three deaths linked to a vaping product
- Indicates at least four deaths linked to a vaping product
- Indicates at least five deaths linked to a vaping product
- Indicates at least six deaths linked to a vaping product.
- Hospitalizations but no confirmed deaths.
For verification see, "Sixty-eight deaths have been confirmed in 29 states and the District of Columbia (as of February 18, 2020): Alabama, California (4), Connecticut, Delaware, District of Columbia, Florida (2), Georgia (6), Illinois (5), Indiana (6), Kansas (2), Kentucky, Louisiana (2), Massachusetts (5), Michigan (3), Minnesota (3), Mississippi, Missouri (2), Montana, Nebraska, New Jersey, New York (4), Oregon (2), Pennsylvania, Rhode Island, South Carolina, Tennessee (2), Texas (4), Utah, Virginia and Washington (2)."[8]
Requests
Discussion on requests
User:VulpesVulpes42, a picture can speak without saying a word.
Discussion on requests
User:PawełMM, I need professional assistance with uploading and/or making modifications for several of the requests below.
CDC image
https://www.cdc.gov/mmwr/volumes/68/wr/mm6808a1.htm?s_cid=mm6808a1_w Image might be useful to upload.
Flickr image
![]() Not done Images not needed.
Not done Images not needed.
https://flickr2commons.toolforge.org/#/
https://flickr.com/photos/elsaolofsson/50923010063/ Extract image of Hemp Bombs CBD Vape Mango Bottle
https://www.flickr.com/photos/vaping360/24281909498/in/album-72157685705737461/
https://www.flickr.com/search/?text=vaping&license=2%2C3%2C4%2C5%2C6%2C9
![]() Not done Images not needed.
Not done Images not needed.
Cherry lime cola packaging and e-liquid bottle only without the scribble graphics design
Proposed file name: File:Cherry Lime Cola Packaging and E-liquid Bottle.png
{{Information
|Description=A photograph of a cherry lime cola packaging and a cherry lime cola e-liquid bottle.
|Source=https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/hina-singh-enterprises-inc-dba-just-eliquids-distro-inc-608444-07202020
|Date=2020-07-20
|Author=United States Food and Drug Administration
|Permission=
|other-versions=
}}
{{PD-USGov-FDA}}
[[Category:FDA food safety inspections]]
[[Category:E-liquid]]
The company name is Just Eliquids Distro Inc.[9] They still have a Facebook page,[10] but the company website has vanished.[11]
The company is back in business with a new product and new packaging called "Cherry Lime Fizz". See, "Formerly known as Cherry Lime Cola and Cola Man."[12][13]
- Request
Upload the cherry lime cola packaging and e-liquid bottle without the scribble design under a new file name. The scribble pattern needs to be removed. The scribble pattern can be changed to solid red. Do not upload "Exhibit B" to the right.
The part where it states Cherry Lime Cola, 3 MG, and 100 ML needs to remain visible. The scribble lines design is what needs to be removed. Replace the black scribble lines with the solid red color.[14]
- Graphist opinion(s)
Electronic cigarette
https://everipedia.org/wiki/lang_en/Electronic_cigarette
{{Information
|Description=The "Electronic cigarette" article at Everipedia
|Source=https://everipedia.org/wiki/lang_en/Electronic_cigarette
|Date=2026-01-30
|Author=English Wikipedia and Everipedia contributors
|Permission=
|other_versions=
}}
{{cc-by-4.0}}
[[Category:Electronic cigarettes]]
CDC videos
https://www.cdc.gov/tobacco/basic_information/e-cigarettes/vapefreeyouth.html
I now take from you your "[ power]". In the name of my father and his father before, I, QuackGuru, cast you out! Whosoever holds this WP:KEY, if they be worthy, shall possess the power of WP:NPOV.
E-cigarette 2.0 It would be an excessive amount of content to keep all the content in the main article. post at the English Wikipedia ANI was misleading because it did not state David is a according to the modern definition of the word. This demonstrates he deserves a topic from and deserves to be desyopped.
https://pubmed.ncbi.nlm.nih.gov/36692176/ PMID: 36692176 PMCID: PMC10152356 (available on 2024-05-01) DOI: 10.5664/jcsm.10428
https://magazine.medlineplus.gov/article/vaping-what-you-need-to-know/
https://commons.wikimedia.org/wiki/Category:Cannabis_vaporizers Desktop Vaporizer
https://flickr.com/search/?text=Cannabis%20vaporizers&license=2%2C3%2C4%2C5%2C6%2C9
https://pubmed.ncbi.nlm.nih.gov/28355118/
https://pubmed.ncbi.nlm.nih.gov/?linkname=pubmed_pubmed_citedin&from_uid=28355118
https://pubmed.ncbi.nlm.nih.gov/33385148/
https://pubmed.ncbi.nlm.nih.gov/33256496/
https://commons.wikimedia.org/wiki/File:Vape-Shop-Elektronicka-Cigareta-Kosice.jpg
Uploading 101 Guide
https://commons.wikimedia.org/wiki/Commons:First_steps
- One can change the background color using the free program GIMP. There are free tutorials online for how to use it. Alternatively, one can head to Commons:Graphic Lab/Illustration workshop and make requests there.
- When using Special:Upload, simply click on "Choose File". and choose one of those many files, and only that one will be uploaded.
- The file needs to be uploaded (commonly called "downloaded") to your own computer first. Screenshotting should only be done as a last resort, because you miss saving the original file, often leading to a loss of quality. Always try to download the original image directly instead. Some images have download buttons for them, and in that case, you should use those. Otherwise, most images on the internet can be downloaded by right-clicking on the image (if you are using a right-handed mouse configuration). This will bring up a menu with options such as "Save image", "Save image as", or similar. Use those.
- Wikimedia Commons has a couple of built-in methods for uploading files. The simplest one is accessed by visiting the page Special:Upload. There, scroll past the information until you see an interface grouped into three sections: "Source file", "File description", and "Upload options". You can safely ignore "Upload options", because its default settings are good, so we will only focus on the first two.
- In the "Source file" section, you should see a small gray button labelled "Choose file" or similar, depending on your browser. To select a file for uploading, you can either drag and drop the desired file onto the button, or click it to bring up your file manager, and select the file from there. Once you have done that, you are done with the "Source file" section and are ready to move on to "File description".
- The field "Destination filename" will be automatically filled in from your chosen file. Simply change it to your liking.
- The field "Summary" contains the following a template:
{{Information
|Description=
|Source=
|Date=
|Author=
|Permission=
|other-versions=
}}
Simply add the correct licensing templates such as {{cc-by-4.0}} underneath the Information template. The "Summary" field might look something like this when you are done, to give you an example:
{{Information
|Description={{en|1=Temperature zones in a combustible cigarette '''(A)''' in comparison to different Heated Tobacco Products '''(B)'''.}}
|Source=https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2019.00287/full
|Date=2019-03-26
|Author=Nadja Mallock, Elke Pieper, Christoph Hutzler, Frank Henkler-Stephani, and Andreas Luch
|Permission=
|other-versions=
}}
{{cc-by-4.0}}
- If you added a licensing template in the previous step, you could skip the "Licensing" field. If not, simply pick the correct one from the list.
- Finally, the "Categories" field. Simply click on the plus symbol, type in the category you want, and click on the "OK" button to add the desired category. If you want to add more than one category, simply repeat the process (note that the plus symbol moves all the way to the right each time). Alternatively, you can add the category as source code in the "Summary" field from before, if you prefer.
- After all that, you are done. Simply click on the "Upload file" (or "Preview" to review) button furthest down on the page.
Template:Creative Commons text attribution notice
https://en.wikipedia.org/wiki/Template:Creative_Commons_text_attribution_notice text available under the CC BY-SA 4.0 license is now compatible
Needs review
https://www.axios.com/2023/04/27/wikipedias-influence-grows
https://commons.wikimedia.org/wiki/Main_Page
WP:LISTN
https://en.wikipedia.org/wiki/Wikipedia:Notability#Stand-alone_lists
WP:CLN
https://en.wikipedia.org/wiki/Wikipedia:Categories,_lists,_and_navigation_templates
WP:AOAL
https://en.wikipedia.org/wiki/Wikipedia:Categories,_lists,_and_navigation_templates#Lists
https://pubmed.ncbi.nlm.nih.gov/18025429/
https://www.malwarefox.com/microsoft-edge-virus/ %temp%
https://www.verywellhealth.com/milk-chocolate-morning-diet-5191422
https://en.wikipedia.org/wiki/Talk:E-liquid Archive 1
https://en.wikipedia.org/wiki/WikiProject_Medicine
Green Tobacco Sickness Dragonite International
https://nida.nih.gov/publications/drugfacts/vaping-devices-electronic-cigarettes
https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/resources/index.html
https://en.wikipedia.org/wiki/Tobacco_harm_reduction#Heat-not-burn_products Needs update.
https://en.wikipedia.org/wiki/Snus Article needs major update.
News articles regarding Wikipedia
[15] The Brutal Edit War Over a 3D Printer’s Wikipedia Page
[16] Unsourced, unreliable, and in your face forever: Wikidata, the future of online nonsense Author Andreas Kolbe
https://www.nationalinjuryadvocates.com/defective-product/
https://en.wikipedia.org/wiki/User:Aaap91597 Contact.
https://en.wikipedia.org/w/index.php?title=Chiropractic&oldid=932374991 https://en.wikipedia.org/w/index.php?title=Chiropractic&diff=prev&oldid=349579183
https://en.wikipedia.org/wiki/Carcinogen#In_cigarettes
https://en.wikipedia.org/wiki/Draft:List_of_drug_prices
https://www.youtube.com/watch?v=LIyzUVfJpN4 Please ask ABC News for screen shot of "I want to start a no-vaping campaign."
A teenager who almost lost her life as a result of vaping is seen here hooked up to a life-supporting ventilator and holding up a handwritten sign. The sign says: "I want to start a no-vaping campaign."
https://www.wsj.com/news/collection/vaping1007-c63cd58a
[2] Need a review that cited this.
References
- ↑ Egel, Corey (24 October 2019). "New Public Education Campaign Targets Deadly Outbreak of Vaping-Related Illness". California Department of Public Health.
- ↑ Tang, Moon-shong; Wu, Xue-Ru; Lee, Hyun-Wook; Xia, Yong; Deng, Fang-Ming; Moreira, Andre L.; Chen, Lung-Chi; Huang, William C.; Lepor, Herbert (2019). "Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice". Proceedings of the National Academy of Sciences. 116 (43): 21727–21731. doi:10.1073/pnas.1911321116. ISSN 0027-8424. PMID 31591243.
false and defamatory defamation of character https://meta.wikimedia.org/wiki/Universal_Code_of_Conduct/Policy_text https://slate.com/technology/2019/07/wikipedia-fram-banning-editor-controversy.html
Check
https://mdwiki.org/wiki/Health_effects_of_tobacco
Check next year: Researchers are doing research to obtain more data regarding e-cigarettes and their usage.[1] This knowledge may result in additional regulations in the US.[1]
- The benefits and health effects remain uncertain
See "Significant uncertainty exists about e-cigarette safety and efficacy, rendering patient discussions about these devices challenging."[17]
See "These devices are unregulated, of unknown safety, and of uncertain benefit in quitting smoking." See "Although research has improved our understanding of e-cigarettes since these initial 2011 recommendations, safety and efficacy remains uncertain."[18]
See "The USPSTF concludes that the current evidence on the use of ENDS for conventional smoking cessation is insufficient. Evidence is lacking and conflicting, and the balance of benefits and harms cannot be determined."[19]
The WP:MEDRS compliant sources confirm that "The benefits and the health effects of e-cigarettes are uncertain."

References
- ↑ 1.0 1.1 "E-Cigarettes". Tobacco Control Research Branch of the National Cancer Institute. 2026.
{{cite web}}: Check date values in:|year=(help) - ↑ "5 Tips to Help Avoid Vape Battery Explosions" (PDF). United States Food and Drug Administration. April 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Text was added to Public health consequences section
Appeal to Young People
E-cigarettes pose potential risks to the population as a whole. E-cigarettes could cause public health harm if they:
- Increase the number of youth and young adults who are exposed to nicotine.
- Lead non-smokers to start smoking conventional cigarettes and other burned tobacco products such as cigars and hookah.
- Sustain nicotine addiction so smokers continue using the most dangerous tobacco products – those that are burned – as well as e-cigarettes, instead of quitting completely.
- Increase the likelihood that former smokers will again become addicted to nicotine by using e-cigarettes, and will start using burned tobacco products again.
Upload image of Public Health Impact section. Click on it to make it purple before uploading image.
References
- ↑ "Did You Know? – Public Health Impact". Surgeon General of the United States. 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Gallery
- E-cigarette related media
-
NIDA Live - The Science of Vaping.
-
Why Teens are Attracted to Vaping.
-
In 2019 and 2020, an outbreak of lung injury was linked to vaping.[1]
-
Dr. Nora Volkow on Addiction - A Disease of Free Will.
-
Julius Dein Performs A Magic Trick on a Vape.
References
- ↑ "Lung Injury Associated with E-cigarette or Vaping Products". Centers for Disease Control and Prevention. 2019.
File needs update

How do e-cigarettes affect the brain?[2] The nicotine in e-liquids readily absorbs into the bloodstream when a person uses an e-cigarette.[2] Upon entering the blood, nicotine stimulates the adrenal glands to release the hormone epinephrine (adrenaline).[2] Epinephrine stimulates the central nervous system and increases blood pressure, breathing, and heart rate.[2] As with most addictive substances, nicotine increases levels of a chemical messenger in the brain called dopamine, which affects parts of the brain that control reward (pleasure from natural behaviors such as eating).[2] These feelings motivate some people to use nicotine again and again, despite possible risks to their health and well-being.[2]

How do e-cigarettes affect the brain?[2] The nicotine in e-liquids readily absorbs into the bloodstream when a person uses an e-cigarette.[2] Upon entering the blood, nicotine stimulates the adrenal glands to release the hormone epinephrine (adrenaline).[2] Epinephrine stimulates the central nervous system and increases blood pressure, breathing, and heart rate.[2] As with most addictive substances, nicotine increases levels of a chemical messenger in the brain called dopamine, which affects parts of the brain that control reward (pleasure from natural behaviors such as eating).[2] These feelings motivate some people to use nicotine again and again, despite possible risks to their health and well-being.[2]
References
- ↑ 1.0 1.1 Di Matteo, Vincenzo; Pierucci, Massimo; Di Giovanni, Giuseppe; Benigno, Arcangelo; Esposito, Ennio (2007). "The Neurobiological Bases for the Pharmacotherapy of Nicotine Addiction". Current Pharmaceutical Design. 13 (12): 1269–1284. doi:10.2174/138161207780618920. ISSN 1381-6128. PMID 17504235.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 "Electronic Cigarettes (E-cigarettes)". National Institute on Drug Abuse. National Institutes of Health. March 2018.
 This article incorporates text from this source, which is in the public domain. Cite error: Invalid
This article incorporates text from this source, which is in the public domain. Cite error: Invalid <ref>tag; name "NIDA2018" defined multiple times with different content
Reward structures of the human brain
 |
 |
| Prior version File:Recolored Overview of reward structures in the human brain2.png |
New version File:Possible effects of nicotine on the developing human brain (cropped).jpg |
This was an old, abandoned request that never was finished. The blue line and the orange line are part of the same structure. It should not have been split into one being orange and the other being blue. That's what caused all the confusion. The original upload was all blue and then it split into two different colors. The undefined blue line was wrong and should have been deleted.
The text is in black for each pathway. It may make more sense to have each text in parenthesis such as (orange) match the same color as the lines.
Mesocortical pathway (orange) Mesolimbic pathway (green) Nigrostriatal pathway (red) Tuberoinfundibular pathway (missing from diagram; maybe blue can work)
The dopamine pathway Tuberoinfundibular pathway () is missing from the structure. The tuberoinfundibular pathway is shown in opaque blue, which connects from hypothalamus to the pituitary gland.[20]
SNc and VTA are not spelled out. They should be spelled out. They are Substantia nigra (SNc) andV entral tegmental area (VTA).
I'm requesting the image to the right be traced with the same line as the image to the left except for the blue line.
https://en.wikipedia.org/wiki/User:QuackGuru/Undefined_blue_line
Fig. 1 and Fig. 2 and Fig. 4
Not compatible with Wikipedia.
See under Copyright and License information:
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- ↑ Seo, An Deok; Kim, Dong Chan; Yu, Hee Joon; Kang, Min Jae (2016). "Accidental ingestion of E-cigarette liquid nicotine in a 15-month-old child: an infant mortality case of nicotine intoxication". Korean Journal of Pediatrics. 59 (12): 490. doi:10.3345/kjp.2016.59.12.490. PMC 5300914. PMID 28194215.
- Flickr images
https://flickr2commons.toolforge.org/#/
https://flickr.com/photos/truthtodare/7512404654/
https://flickr.com/photos/kthtrnr/11896090724/
https://flickr.com/photos/53216876@N00/25742200974/
https://flickr.com/photos/53216876@N00/26074189640/
https://flickr.com/photos/53216876@N00/26074196280/
https://flickr.com/photos/gorillazs-photographer/40187037820/
Vein art
- Vein art
-
English Wikipedia article Usage of electronic cigarettes was deleted
The title Usage of electronic cigarettes is specifically about content related to prevalence, frequency, rates of use, and other things about the usage of e-cigarettes. It is not about the term vaping in general. It is not about the broader term of vaping general.This is the same case as the terminology smoking. For example, the English Wikipedia has an article on Smoking, and that article has a section on prevalence of tobacco use.[22] There is a link in that section to a subpage on prevalence of tobacco use[23] The topic Smoking and the spin-off called prevalence of tobacco use are two different topics. The English Wikipedia article called Electronic cigarette[24] is a general topic on e-cigarette, while the spin-off on the Usage of electronic cigarettes[25] is a specific topic on its prevalence, frequency, rates of use, motivation, and so on.
The content on usage of electronic cigarettes became too large to cover all the content on the topic in the main Electronic cigarette article on the English Wikipedia. So, QuackGuru created a spin-off article on the topic on 22:20, 4 June 2019.[26] The Usage of electronic cigarettes contains content that is not found in the main Electronic cigarette article on the English Wikipedia including the sections International, United States, United States youth, European Union, European Union youth, United Kingdom, Australia, and Other uses. The main Electronic cigarette article on the English Wikipedia does not have all the previously mentioned sections that are found in Usage of electronic cigarettes article. No evidence was presented it was arguably a POVFork or ContentFork of a recreation of an existing topic. On the contrary, the evidence shows it is a spin-off article on a notable topic with well over a hundred reliable sources on the topic.
Source content replaced with the unsourced content "Younger users also experiment with e-cigarettes." on 10:56, 13 August 2017.[27]


