Superphenalene
 Chemical structure of superphenalene
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dibenzo[u′v′,a′1b′1]benzo[4′′,10′′]anthra[3′′,2′′,1′′,9′′,8′′:1′,12′,11′,10′]tetrapheno[5′,6′,7′,8′,9′:4,5,6,7]tetraceno[2,1,12,11,10,9-uvwxyza1b1]hexaceno[2,1,16,15,14,13,12,11-defghijklmno:3,4,5,6,7,8,9,10-d′e′f′g′h′i′j′k′l′m′n′o′]diheptacene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| C96H30 | |
| Molar mass | 1183.296 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Superphenalene is a very large polycyclic aromatic hydrocarbon (PAH) with chemical formula C96H30. It can be formally considered to consist of three fused superbenzenes (hexa-peri-hexabenzocoronene).[1]
It can be considered as an overlapping structure of three hexa-peri-hexabenzocoronenes arranged symmetrically around a center.[2][3] These have also been known as building blocks of molecular electronics since 2004 as they form self-assembling columns and nanotubes.[4]
It and its hexa-tert-butyl derivative were first prepared in 1997, along with a collection of other very large PAHs. Due to the low solubility of these compounds, they could only be characterized by mass spectrometry when they were first synthesized, as neither NMR nor UV-Vis data could be collected.[5]
Occurrence
It is not known to occur naturally.
Properties
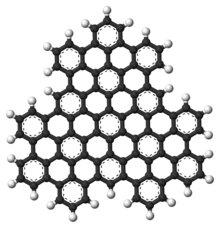
Superphenalene has a planar geometry. With 540,000 mesomeric boundary structures, it has significantly more than hexabenzocoronene (250), supernaphthalene (16,100) and also buckminsterfullerene (12,500).[1] The molecule has a threefold symmetry axis perpendicular to the molecule (C3).
References
- ^ a b Randić, Milan; Gao, Xiaofeng (1999). "Giant benzenoid hydrocarbons. Superphenalene resonance energy". New Journal of Chemistry. 23 (2): 251–260. doi:10.1039/A808949C.
- ^ Ito, Shunji; Herwig, Peter Tobias; Böhme, Thilo; Rabe, Jürgen P.; Rettig, Wolfgang; Müllen, Klaus (2000). "Bishexa-peri-hexabenzocoronenyl: A 'superbiphenyl'". Journal of the American Chemical Society. 122 (32): 7698–7706. doi:10.1021/ja000850e.
- ^ Wu, Jishan; Watson, Mark D.; Tchebotareva, Natalia; Wang, Zhaohui; Müllen, Klaus (2004). "Oligomers of hexa-peri-hexabenzocoronenes as 'super-oligophenylenes': Synthesis, electronic properties, and self-assembly". The Journal of Organic Chemistry. 69 (24): 8194–8204. doi:10.1021/jo0490301. PMID 15549787.
- ^ Hill, J. P.; Jin, W.; Kosaka, A.; Fukushima, T.; Ichihara, H.; Shimomura, T.; Ito, K.; Hashizume, T.; Ishii, N.; Aida, T. (2004). "Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube". Science. 304 (5676): 1481–1483. Bibcode:2004Sci...304.1481H. doi:10.1126/science.1097789. PMID 15178796. S2CID 39674411.
- ^ Iyer, Vivekanantan S.; Wehmeier, Mike; Brand, J. Diedrich; Keegstra, Menno A.; Müllen, Klaus (1997-08-18). "From Hexa‐ peri ‐hexabenzocoronene to "Superacenes"". Angewandte Chemie International Edition in English. 36 (15): 1604–1607. doi:10.1002/anie.199716041. ISSN 0570-0833.
- Chemicals without a PubChem CID
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Polycyclic aromatic hydrocarbons