Maralixibat
 | |
| Names | |
|---|---|
| Trade names | Livmarli |
| Other names | LUM001 |
| |
| Clinical data | |
| Drug class | Ileal bile acid transporter (IBAT) inhibitor |
| Main uses | Itchiness due to high bile salts in Alagille syndrome[1] |
| Side effects | Diarrhea, abdominal pain, low fat soluble vitamins, liver problems, gastrointestinal bleeding, bone fractures[1] |
| Routes of use | By mouth |
| Typical dose | 380 mcg/kg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
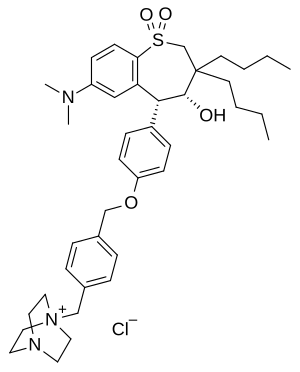
| Formula | C40H56ClN3O4S |
| Molar mass | 710.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Maralixibat, sold under the brand name Livmarli, is a medication used to treat itchiness due to high bile salts in people with Alagille syndrome.[1] It is used in people over the age of 2 month to 1 year.[2][3] It is taken by mouth.[4]
Commons side effects include diarrhea, abdominal pain, low fat soluble vitamins, liver problems, gastrointestinal bleeding, and bone fractures.[1] It is believed to be safe in pregnancy.[3] It is an ileal bile acid transporter (IBAT) inhibitor.[1]
Maralixibat was approved for medical use in the United States in 2021.[1][5] In Europe it is available as an orphan drug.[4] In the United States 30 ml of 9.5 mg/mL solution costs about 54,000 USD as of 2022.[6]
Medical uses
It is started at a dose of 190 mcg/kg for one week and increased to 380 mcg/kg.[1]
Mechanism

In terms of the mode of action we find that Maralixiba works via the inhibition of IBAT, this in turn lowers the reabsorption of bile acids in ileum. As a consequence an increased elimination of bile acids occurs via feces [7]
History
Maralixibat was granted orphan drug designations in 2013,[8][9] and in 2020.[10]
Society and culture
Legal status
In October 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization under exceptional circumstances for the medicinal product Livmarli, intended for the treatment of cholestatic pruritus in patients with Alagille syndrome (ALGS).[11] The applicant for this medicinal product is Mirum Pharmaceuticals International B.V.[11]
Names
Maralixibat chloride is the international nonproprietary name (INN).[12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Livmarli- maralixibat chloride solution". DailyMed. Archived from the original on 1 November 2021. Retrieved 31 October 2021.
- ↑ "Livmarli". Archived from the original on 25 October 2022. Retrieved 26 October 2022.
- ↑ 3.0 3.1 "Maralixibat Monograph for Professionals". Drugs.com. Retrieved 26 October 2022.
- ↑ 4.0 4.1 "Maralixibat". SPS - Specialist Pharmacy Service. 18 July 2019. Archived from the original on 25 September 2021. Retrieved 26 October 2022.
- ↑ "Maralixibat: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 30 September 2021. Retrieved 29 September 2021.
- ↑ "Livmarli Prices, Coupons, Copay & Patient Assistance". Drugs.com. Retrieved 26 October 2022.
- ↑ 7.0 7.1 Komaniecka, Nina; Maroszek, Sonia; Drozdzik, Maria; Oswald, Stefan; Drozdzik, Marek (January 2024). "Transporter Proteins as Therapeutic Drug Targets—With a Focus on SGLT2 Inhibitors". International Journal of Molecular Sciences. 25 (13): 6926. doi:10.3390/ijms25136926. ISSN 1422-0067. Archived from the original on 2024-07-03. Retrieved 2024-07-02.
- ↑ "Maralixibat Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 4 September 2013. Archived from the original on 30 September 2021. Retrieved 29 September 2021.
- ↑ "Maralixibat Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 4 September 2013. Archived from the original on 30 September 2021. Retrieved 29 September 2021.
- ↑ "Maralixibat Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 21 October 2020. Archived from the original on 30 September 2021. Retrieved 29 September 2021.
- ↑ 11.0 11.1 "Livmarli: Pending EC decision". European Medicines Agency. 14 October 2022. Archived from the original on 25 October 2022. Retrieved 15 October 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 75". WHO Drug Information. 30 (1). hdl:10665/331046.
External links
| Identifiers: |
|---|
- "Maralixibat". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-09-30. Retrieved 2022-10-15.
- "Maralixibat chloride". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-10-01. Retrieved 2022-10-15.
- Clinical trial number NCT02160782 for "Safety and Efficacy Study of LUM001 (Maralixibat) With a Drug Withdrawal Period in Participants With Alagille Syndrome (ALGS) (ICONIC)" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs acting on the gastrointestinal system and metabolism
- Orphan drugs
- Heterocyclic compounds with 2 rings
- Sulfur heterocycles
- Nitrogen heterocycles
- Quaternary ammonium compounds
- Tertiary amines
- RTT
- All stub articles
- Gastrointestinal system drug stubs