High-grade serous carcinoma of the ovary
| Ovarian cancer | |
|---|---|
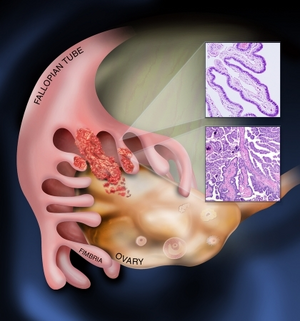 | |
| Diagram showing fimbrial end of the fallopian tube and spread to the ovarian surface epithelium | |
| Specialty | Oncology, gynecology |
| Symptoms | Large tummy or mass[1] |
| Usual onset | Age 65-years[2] |
| Causes | Unknown[2] |
| Diagnostic method | Raised CA125[2] |
High-grade serous carcinoma of the ovary (HGSCO) is a type of cancer that presents in an ovary.[2][3] It typically occurs in early postmenopausal females, and frequently presents in the late stage of the condition, usually with a large tummy or mass.[1]
HGSCO is a type of serous tumor of the ovary that typically occurs in women aged over 60-years, who have a family history of ovarian or breast cancer and who have never had children.[2] Having multiple births and fewer menses in life, breastfeeding, and taking the oral contraceptive pill, appear to give protection.[2] Although originally thought to arise from the squamous epithelial cell layer covering the ovary, it is now established that most HGSCOs originate in a precursor lesion called serous tubal intraepithelial carcinoma, found in the finger-like projections of the fallopian tube.[2]
Around 70% of ovarian cancers are of the HGSCO type.[2]
Risk factors
Environmental risk factors
The ‘incessant ovulation’ theory is suggested by the strong correlation between the number of ovulatory cycles of an individual and their risk of ovarian cancer.[4]
This trend is reflected in the protective effects of pregnancy, parity and breastfeeding against ovarian cancer,[5][6] and similar findings in epidemiological studies that have indicated a reduction of risk associated with use of oral contraceptive pills.[7][4]
Ovulation is accepted as the cause of ovarian cortical inclusion cysts, the precursor lesions of serous carcinomas, and lower numbers of these cortical inclusion cysts are thought to be the mechanism by which reducing lifetime ovulations can lower the risk of developing HGSC.[8]
Conversely, a temporal association with menopausal hormone therapy and incidence of HGSC was found,[9] and polycystic ovarian syndrome (PCOS) was shown to contribute to a doubling of the risk of ovarian cancer.[10]
Endometriosis can increase risk for other ovarian cancer subtypes, but is not associated with HGSC.[11]
Genetic risk factors
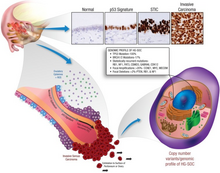
More than 20% of ovarian cancer tumours have hereditary origin. The majority of these feature mutations in the tumour suppressor BRCA genes, which tend to give rise to HGSC.[12] A mutation in BRCA1 or BRCA2 can confer a lifetime ovarian cancer risk of 40-50% and 10-20% respectively,[13] with BRCA2 mutations strongly associated with better clinical outcomes.
A specific tumour protein 53 (TP53) expression pattern in the fallopian tube epithelium – the ‘p53 signature’ - is thought to be a precursor marker of HGSC.[14] TP53-/- mice (in which the TP53 gene has been deleted) do not develop ovarian carcinomas.[15] However, TP53 mutations were found in 96% of HGSC cases.[16] A local abnormal TP53 expression may thus be indicative of HGSC.[17] In women, pelvic HGSC show either a complete absence of P53 expression, or overexpression, suggesting that any aberration of P53 leads to tumour development.[18] Additionally, overexpression of TP53 is associated with better clinical outcome whereas an absence of the p53 protein is linked to an increased risk of HGSC tumour recurrence.[18]
A recent mouse model suggest that a p53 mutation may induce HGSC arising from the ovary rather than the Fallopian tube.[19]
Diagnosis
Symptoms include persistent bloating, postmenopausal bleeding, and/or appetite loss.[20]
Transvaginal ultrasonography as well as cancer marker CA125 level analysis is often used to determine potential malignancy of suspect pelvic masses.[21]
Surgical staging is the procedure by which the abdominal cavity and lymph nodes are examined for malignant tissue, usually via laparoscopy. Tissue biopsies may be taken for further analysis. It is not until this histological analysis stage that actual diagnosis of HGSC can be made.[21]
If glands are seen to fuse with intricate, extensive papillae featuring epithelial tufting with solid nests surrounded by a space alongside irregular slit-like spaces, then serous carcinoma is suspected.
Distinguishing between LGSC and HGSC:[22]
- Necrosis is common in HGSC and absent in LGSC, as are giant (multi- or mononucleated) tumour cells.
- Psammoma bodies are more frequent in low-grade serous carcinoma.
- Tp53 expression is assessed for mutations, overexpression or absence – common features of high-grade serous carcinomas.
- LGSCs are generally limited to micropapillary growth patterns, whereas HGSCs can exhibit admixed patterns.
Distinction of HGSC from high-grade endometrioid carcinoma is not always possible.[22]
The progression of HGSC may also be determined from examining the cadherin expression profile.[23]
Screening
As ovarian cancer is rarely symptomatic until an advanced stage,[24] regular pre-emptive screening is a particularly important tool for avoiding the late stage at which most patients present. However, A 2011 US study found that transvaginal ultrasound and cancer marker CA125 screening did not reduce ovarian cancer mortality.[25] In contrast, a more recent UK study found that up to 20% of ovarian cancer deaths could be prevented through annual performance of these procedures.[26]
Prevention
Prevention for an individual deemed at risk of HGSC has, up until recently, been (bilateral or unilateral) removal of both the ovary and the Fallopian tube (salpingo-oophorectomy).
With hormonal and even morbidity issues resulting from ovary removal, and the increased evidence for the role of the Fallopian tubes HGSC pathogenesis, optimisation of this procedure has been to remove just the Fallopian tube(s) (salpingectomy) with the ovaries remaining until age of menopause [27][28] - although critics of this argue that a reduced blood supply to the ovaries may induce premature menopause regardless.[29]
Prophylactic salpingo-oophorectomy is frequently performed in carriers of BRCA1 or BRCA2 mutations,[30] although the benefits conferred by this procedure may vary dependent on the specific mutation.[31]
Tubal ligation is a less invasive prophylactic treatment shown to significantly reduce the risk of HGSC.[32]
Treatment
Cytoreductive "debulking" surgery may be performed prior to chemotherapy treatment in order to decrease the physical mass of the tumour and thus reduce the number of chemotherapy cycles needed.[21] The typical advanced presentation as well as extra-ovarian spread seen in HGSC can require aggressive debulking procedures.[33] In some cases total abdominal hysterectomy will be performed, in other cases where the patient intends to bear children a salpingo-oophorectomy is performed instead.
Typical chemotherapy is six cycles of intraperitoneally-delivered platinum-base adjuvant chemotherapy with agents such as carboplatin.[21] Measurements of blood CA125 levels are used to determine patient response to the treatment.
Between 20% and 30% of patients relapse within six months of treatment.[34]
Poly ADP ribose polymerase (PARP) inhibitors are another possible treatment, with carriers of BRCA1/2 mutations being the most responsive [35][36]
Epidemiology
A study of incidence rates in the US between 1992 and 1999 found that the age-specific incidence rate for HGSC doubles every 10 years up until age 55, where it plateaus at approximately 20 cases per 100,000 women - before dropping dramatically after age 75.[37]
Ovarian cancer incidence rates are low in East Asia[38] and highest in Europe, the United States, and Australia/New Zealand.[39]
Since 1975, survival rates for ovarian cancer have steadily improved with a mean decrease of 51% by 2006 of risk of death from ovarian cancer for an advanced stage tumour.[40] The increase has mainly been due to successful extended life expectancy of affected patients rather than an improvement in cure rates.
A racial disparity exists between black and white women in the US, where black women experience a higher mortality risk from ovarian cancer.[40][41]
See also
References
- ↑ 1.0 1.1 Rashid, Sameera; Arafah, Maria A.; Akhtar, Mohammed (1 May 2022). "The Many Faces of Serous Neoplasms and Related Lesions of the Female Pelvis: A Review". Advances in Anatomic Pathology. 29 (3): 154–167. doi:10.1097/PAP.0000000000000334. ISSN 1533-4031. Archived from the original on 29 July 2022. Retrieved 29 July 2022.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 WHO Classification of Tumours Editorial Board, ed. (2020). "1. Tumours of the ovary". Female genital tumours: WHO Classification of Tumours. Vol. 4 (5th ed.). Lyon (France): International Agency for Research on Cancer. pp. 45–47. ISBN 978-92-832-4504-9. Archived from the original on 2022-06-17. Retrieved 2022-07-01.
- ↑ Shih, Ie-Ming; Wang, Yeh; Wang, Tian-Li (January 2021). "The Origin of Ovarian Cancer Species and Precancerous Landscape". The American Journal of Pathology. 191 (1): 26–39. doi:10.1016/j.ajpath.2020.09.006. ISSN 1525-2191. PMID 33011111. Archived from the original on 2022-06-15. Retrieved 2022-07-05.
- ↑ 4.0 4.1 Fathalla, M. F. (2013-01-01). "Incessant ovulation and ovarian cancer - a hypothesis re-visited". Facts, Views & Vision in ObGyn. 5 (4): 292–297. ISSN 2032-0418. PMC 3987381. PMID 24753957.
- ↑ Risch, H. A.; Marrett, L. D.; Howe, G. R. (1994-10-01). "Parity, contraception, infertility, and the risk of epithelial ovarian cancer". American Journal of Epidemiology. 140 (7): 585–597. doi:10.1093/oxfordjournals.aje.a117296. ISSN 0002-9262. PMID 7942759.
- ↑ Gwinn, M. L.; Lee, N. C.; Rhodes, P. H.; Layde, P. M.; Rubin, G. L. (1990-01-01). "Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer". Journal of Clinical Epidemiology. 43 (6): 559–568. doi:10.1016/0895-4356(90)90160-q. ISSN 0895-4356. PMID 2348208. Archived from the original on 2021-07-10. Retrieved 2021-12-26.
- ↑ Kurman, R. J. (2013-12-01). "Origin and molecular pathogenesis of ovarian high-grade serous carcinoma". Annals of Oncology. 24 Suppl 10: x16–21. doi:10.1093/annonc/mdt463. ISSN 1569-8041. PMID 24265397.
- ↑ DastranjTabrizi, Ali; MostafaGharabaghi, Parvin; SheikhzadehHesari, Farzam; Sadeghi, Liela; Zamanvandi, Sharareh; Sarbakhsh, Parvin; Ghojazadeh, Morteza (2016-01-01). "Impact and mechanistic role of oral contraceptive pills on the number and epithelial type of ovarian cortical inclusion cysts; a clinicopathology and immunohistochemical study". Diagnostic Pathology. 11: 30. doi:10.1186/s13000-016-0482-6. ISSN 1746-1596. PMC 4802821. PMID 27000861.
- ↑ Yang, Hannah P.; Anderson, William F.; Rosenberg, Philip S.; Trabert, Britton; Gierach, Gretchen L.; Wentzensen, Nicolas; Cronin, Kathleen A.; Sherman, Mark E. (2013-06-10). "Ovarian cancer incidence trends in relation to changing patterns of menopausal hormone therapy use in the United States". Journal of Clinical Oncology. 31 (17): 2146–2151. doi:10.1200/JCO.2012.45.5758. ISSN 1527-7755. PMC 3731982. PMID 23650423.
- ↑ Schildkraut, J. M.; Schwingl, P. J.; Bastos, E.; Evanoff, A.; Hughes, C. (1996-10-01). "Epithelial ovarian cancer risk among women with polycystic ovary syndrome". Obstetrics and Gynecology. 88 (4 Pt 1): 554–559. doi:10.1016/0029-7844(96)00226-8. ISSN 0029-7844. PMID 8841217. S2CID 22995413.
- ↑ Pearce, Celeste Leigh; Templeman, Claire; Rossing, Mary Anne; Lee, Alice; Near, Aimee M.; Webb, Penelope M.; Nagle, Christina M.; Doherty, Jennifer A.; Cushing-Haugen, Kara L. (2012-04-01). "Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies". The Lancet. Oncology. 13 (4): 385–394. doi:10.1016/S1470-2045(11)70404-1. ISSN 1474-5488. PMC 3664011. PMID 22361336.
- ↑ Toss, Angela; Tomasello, Chiara; Razzaboni, Elisabetta; Contu, Giannina; Grandi, Giovanni; Cagnacci, Angelo; Schilder, Russell J.; Cortesi, Laura (2015-01-01). "Hereditary ovarian cancer: not only BRCA 1 and 2 genes". BioMed Research International. 2015: 341723. doi:10.1155/2015/341723. ISSN 2314-6141. PMC 4449870. PMID 26075229.
- ↑ Liu, Guoyan; Yang, Da; Sun, Yan; Shmulevich, Ilya; Xue, Fengxia; Sood, Anil K.; Zhang, Wei (2012-10-01). "Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer". Pharmacogenomics. 13 (13): 1523–1535. doi:10.2217/pgs.12.137. ISSN 1744-8042. PMC 3603383. PMID 23057551.
- ↑ Stasenko, Marina; Fillipova, Olga; Tew, William P. (July 2019). "Fallopian Tube Carcinoma". Journal of Oncology Practice. 15 (7): 375–382. doi:10.1200/JOP.18.00662. ISSN 1554-7477. Archived from the original on 2024-03-30. Retrieved 2024-03-30.
- ↑ Liu, Ying; Yen, Hai-Yun; Austria, Theresa; Pettersson, Jonas; Peti-Peterdi, Janos; Maxson, Robert; Widschwendter, Martin; Dubeau, Louis (2015-10-01). "A Mouse Model That Reproduces the Developmental Pathways and Site Specificity of the Cancers Associated With the Human BRCA1 Mutation Carrier State". EBioMedicine. 2 (10): 1318–1330. doi:10.1016/j.ebiom.2015.08.034. ISSN 2352-3964. PMC 4634618. PMID 26629527.
- ↑ Cancer Genome Atlas Research Network (2011-06-30). "Integrated genomic analyses of ovarian carcinoma". Nature. 474 (7353): 609–615. doi:10.1038/nature10166. ISSN 1476-4687. PMC 3163504. PMID 21720365.
- ↑ Ahmed, Ahmed Ashour; Etemadmoghadam, Dariush; Temple, Jillian; Lynch, Andy G.; Riad, Mohamed; Sharma, Raghwa; Stewart, Colin; Fereday, Sian; Caldas, Carlos (2010-05-01). "Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary". The Journal of Pathology. 221 (1): 49–56. doi:10.1002/path.2696. ISSN 1096-9896. PMC 3262968. PMID 20229506.
- ↑ 18.0 18.1 Köbel, Martin; Reuss, Alexander; du Bois, Andreas; Kommoss, Stefan; Kommoss, Friedrich; Gao, Dongxia; Kalloger, Steve E.; Huntsman, David G.; Gilks, C. Blake (2010-10-01). "The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas". The Journal of Pathology. 222 (2): 191–198. doi:10.1002/path.2744. ISSN 1096-9896. PMID 20629008. S2CID 35591123.
- ↑ Kim, Jaeyeon; Coffey, Donna M.; Ma, Lang; Matzuk, Martin M. (2015-06-01). "The ovary is an alternative site of origin for high-grade serous ovarian cancer in mice". Endocrinology. 156 (6): 1975–1981. doi:10.1210/en.2014-1977. ISSN 1945-7170. PMC 5393339. PMID 25815421. Archived from the original on 2022-07-10. Retrieved 2021-12-26.
- ↑ Bankhead, C. R.; Collins, C.; Stokes-Lampard, H.; Rose, P.; Wilson, S.; Clements, A.; Mant, D.; Kehoe, S. T.; Austoker, J. (2008-07-01). "Identifying symptoms of ovarian cancer: a qualitative and quantitative study". BJOG: An International Journal of Obstetrics and Gynaecology. 115 (8): 1008–1014. doi:10.1111/j.1471-0528.2008.01772.x. ISSN 1471-0528. PMC 2607526. PMID 18651882.
- ↑ 21.0 21.1 21.2 21.3 Colombo, N.; Peiretti, M.; Parma, G.; Lapresa, M.; Mancari, R.; Carinelli, S.; Sessa, C.; Castiglione, M.; ESMO Guidelines Working Group (2010-05-01). "Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of Oncology. 21 Suppl 5: v23–30. doi:10.1093/annonc/mdq244. ISSN 1569-8041. PMID 20555088.
- ↑ 22.0 22.1 Vang, Russell; Shih, Ie-Ming; Kurman, Robert J. (2009-09-01). "Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems". Advances in Anatomic Pathology. 16 (5): 267–282. doi:10.1097/PAP.0b013e3181b4fffa. ISSN 1533-4031. PMC 2745605. PMID 19700937.
- ↑ Quattrocchi, Livia; Green, Andrew R.; Martin, Stewart; Durrant, Lindy; Deen, Suha (2011-07-01). "The cadherin switch in ovarian high-grade serous carcinoma is associated with disease progression". Virchows Archiv. 459 (1): 21–29. doi:10.1007/s00428-011-1082-1. ISSN 1432-2307. PMID 21509572. S2CID 1779853. Archived from the original on 2022-07-10. Retrieved 2021-12-26.
- ↑ Nagle, Christina M.; Francis, Jane E.; Nelson, Anne E.; Zorbas, Helen; Luxford, Karen; de Fazio, Anna; Fereday, Sian; Bowtell, David D.; Green, Adèle C. (2011-06-01). "Reducing time to diagnosis does not improve outcomes for women with symptomatic ovarian cancer: a report from the Australian Ovarian Cancer Study Group". Journal of Clinical Oncology. 29 (16): 2253–2258. doi:10.1200/JCO.2010.32.2164. ISSN 1527-7755. PMID 21537035.
- ↑ Buys, Saundra S.; Partridge, Edward; Black, Amanda; Johnson, Christine C.; Lamerato, Lois; Isaacs, Claudine; Reding, Douglas J.; Greenlee, Robert T.; Yokochi, Lance A. (2011-06-08). "Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial". JAMA. 305 (22): 2295–2303. doi:10.1001/jama.2011.766. ISSN 1538-3598. PMID 21642681.
- ↑ Jacobs, Ian J.; Menon, Usha; Ryan, Andy; Gentry-Maharaj, Aleksandra; Burnell, Matthew; Kalsi, Jatinderpal K.; Amso, Nazar N.; Apostolidou, Sophia; Benjamin, Elizabeth (2016-03-05). "Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial". Lancet. 387 (10022): 945–956. doi:10.1016/S0140-6736(15)01224-6. ISSN 1474-547X. PMC 4779792. PMID 26707054.
- ↑ Oliver Perez, M. Reyes; Magriñá, Javier; García, Alvaro Tejerizo; Jiménez Lopez, Jesus Salvador (2015-12-01). "Prophylactic salpingectomy and prophylactic salpingoophorectomy for adnexal high-grade serous epithelial carcinoma: A reappraisal". Surgical Oncology. 24 (4): 335–344. doi:10.1016/j.suronc.2015.09.008. ISSN 1879-3320. PMID 26690823.
- ↑ Daly, Mary B.; Dresher, Charles W.; Yates, Melinda S.; Jeter, Joanne M.; Karlan, Beth Y.; Alberts, David S.; Lu, Karen H. (2015-05-01). "Salpingectomy as a means to reduce ovarian cancer risk". Cancer Prevention Research (Philadelphia, Pa.). 8 (5): 342–348. doi:10.1158/1940-6207.CAPR-14-0293. ISSN 1940-6215. PMC 4417454. PMID 25586903.
- ↑ Pölcher, Martin; Hauptmann, Steffen; Fotopoulou, Christina; Schmalfeldt, Barbara; Meinhold-Heerlein, Ivo; Mustea, Alexander; Runnebaum, Ingo; Sehouli, Jalid (2015-07-01). "Opportunistic salpingectomies for the prevention of a high-grade serous carcinoma: a statement by the Kommission Ovar of the AGO". Archives of Gynecology and Obstetrics. 292 (1): 231–234. doi:10.1007/s00404-015-3697-y. ISSN 1432-0711. PMID 25914073. S2CID 24396058.
- ↑ Kauff, Noah D.; Satagopan, Jaya M.; Robson, Mark E.; Scheuer, Lauren; Hensley, Martee; Hudis, Clifford A.; Ellis, Nathan A.; Boyd, Jeff; Borgen, Patrick I. (2002-05-23). "Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation". The New England Journal of Medicine. 346 (21): 1609–1615. doi:10.1056/NEJMoa020119. ISSN 1533-4406. PMID 12023992.
- ↑ Kauff, Noah D.; Domchek, Susan M.; Friebel, Tara M.; Robson, Mark E.; Lee, Johanna; Garber, Judy E.; Isaacs, Claudine; Evans, D. Gareth; Lynch, Henry (2008-03-10). "Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study". Journal of Clinical Oncology. 26 (8): 1331–1337. doi:10.1200/JCO.2007.13.9626. ISSN 1527-7755. PMC 3306809. PMID 18268356.
- ↑ Sieh, Weiva; Salvador, Shannon; McGuire, Valerie; Weber, Rachel Palmieri; Terry, Kathryn L.; Rossing, Mary Anne; Risch, Harvey; Wu, Anna H.; Webb, Penelope M. (2013-04-01). "Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies". International Journal of Epidemiology. 42 (2): 579–589. doi:10.1093/ije/dyt042. ISSN 1464-3685. PMC 3619957. PMID 23569193.
- ↑ Ferguson, Donna; Han, Lucy (2015). "The role of the fallopian tube in ovarian serous carcinogenesis: biologic mechanisms and clinical impacts" (PDF). American Journal of Clinical and Experimental Obstetrics and Gynecology. 2 (1). Archived (PDF) from the original on 2016-09-16. Retrieved 2021-12-26.
- ↑ Berns, Els M. J. J.; Bowtell, David D. (2012-06-01). "The changing view of high-grade serous ovarian cancer". Cancer Research. 72 (11): 2701–2704. doi:10.1158/0008-5472.CAN-11-3911. ISSN 1538-7445. PMID 22593197. Archived from the original on 2022-07-10. Retrieved 2021-12-26.
- ↑ Ivy, S. Percy; Liu, Joyce F.; Lee, Jung-Min; Matulonis, Ursula A.; Kohn, Elise C. (2016-05-01). "Cediranib, a pan-VEGFR inhibitor, and olaparib, a PARP inhibitor, in combination therapy for high grade serous ovarian cancer". Expert Opinion on Investigational Drugs. 25 (5): 597–611. doi:10.1517/13543784.2016.1156857. ISSN 1744-7658. PMID 26899229. S2CID 25959899. Archived from the original on 2022-07-10. Retrieved 2021-12-26.
- ↑ Parkes, Eileen E.; Kennedy, Richard D. (2016-05-01). "Clinical Application of Poly(ADP-Ribose) Polymerase Inhibitors in High-Grade Serous Ovarian Cancer". The Oncologist. 21 (5): 586–593. doi:10.1634/theoncologist.2015-0438. ISSN 1549-490X. PMC 4861365. PMID 27022037.
- ↑ Quirk, Jeffrey T.; Natarajan, Nachimuthu; Mettlin, Curtis J. (2005-10-01). "Age-specific ovarian cancer incidence rate patterns in the United States". Gynecologic Oncology. 99 (1): 248–250. doi:10.1016/j.ygyno.2005.06.052. ISSN 0090-8258. PMID 16095676.
- ↑ Liede, Alexander; Narod, Steven A. (2002-12-01). "Hereditary breast and ovarian cancer in Asia: genetic epidemiology of BRCA1 and BRCA2". Human Mutation. 20 (6): 413–424. doi:10.1002/humu.10154. ISSN 1098-1004. PMID 12442265.
- ↑ Ferlay, Jacques; Shin, Hai-Rim; Bray, Freddie; Forman, David; Mathers, Colin; Parkin, Donald Maxwell (2010-12-15). "Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008". International Journal of Cancer. 127 (12): 2893–2917. doi:10.1002/ijc.25516. ISSN 1097-0215. PMID 21351269. S2CID 23583962.
- ↑ 40.0 40.1 Temkin, Sarah M.; Terplan, Mishka (2015-10-01). "Trends in Relative Survival for Ovarian Cancer From 1975-2011". Obstetrics and Gynecology. 126 (4): 898. doi:10.1097/AOG.0000000000001073. ISSN 1873-233X. PMID 26393445. S2CID 32185049.
- ↑ Terplan, Mishka; Schluterman, Nicholas; McNamara, Erica J.; Tracy, J. Kathleen; Temkin, Sarah M. (2012-04-01). "Have racial disparities in ovarian cancer increased over time? An analysis of SEER data". Gynecologic Oncology. 125 (1): 19–24. doi:10.1016/j.ygyno.2011.11.025. ISSN 1095-6859. PMID 22108636.