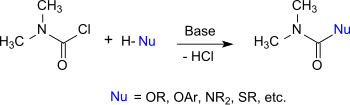Dimethylcarbamoyl chloride

| |
| Names | |
|---|---|
| Preferred IUPAC name
Dimethylcarbamoyl chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.099 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6ClNO | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans,[1] dimethylcarbamoyl chloride can only be used under stringent safety precautions.
Production and occurrence
The production of dimethylcarbamoyl chloride from phosgene and dimethylamine was reported as early as 1879 (reported as "Dimethylharnstoffchlorid" – dimethylurea chloride).[2]
DMCC can be produced in high yields (90%) at 275 °C by reacting phosgene with gaseous dimethylamine in a flow reactor.[3] To suppress the formation of ureas, excess phosgene is used (in a 3:1 ratio).
The reaction can also be carried out at the laboratory scale with diphosgene or triphosgene and an aqueous dimethylamine solution in the two-phase system of benzene–xylene and water in a stirred reactor with sodium hydroxide as an acid scavenger. However, considerably lower yields (56%) are achieved due to the hydrolysis sensitivity of DMCC.[4]
Dimethylcarbamoyl chloride is also formed (together with methyl chloride) when reacting phosgene with trimethylamine.[5]
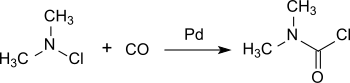
A more recent process is based on chlorodimethylamine, which is converted practically quantitatively to dimethylcarbamoyl chloride on a palladium catalyst under pressure with carbon monoxide at room temperature.[6]
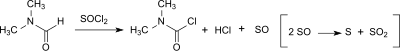
DMCC can also be formed in small amounts (up to 20 ppm) from dimethylformamide (DMF) in the Vilsmeier–Haack reaction[7] or when DMF is used as a catalyst in the reaction of carboxylic acids with thionyl chloride to the corresponding acyl chloride.[8]
The tendency towards DMCC formation depends on the chlorination reagent (thionyl chloride > oxalyl chloride > phosphorus oxychloride) and is higher in the presence of a base. However, dicarbamoyl chloride hydrolyses very quickly to dimethylamine, hydrochloric acid and carbon dioxide (with a half-life of about 6 minutes at 0 °C) so that less than 3 ppm of dicarbamoyl chloride is found in the Vilsmeier product after aqueous workup.[9]
Properties
Dimethylcarbamoyl chloride is a clear, colorless, corrosive and flammable liquid with a pungent odor and a tear-penetrating effect, which decomposes rapidly in water.[10] Because of its unpleasant, toxic, mutagenic and carcinogenic properties,[11][12] it has to be used under extreme precautions.
DMCC behaves like an acyl chloride whose chlorine atom can be exchanged for other nucleophiles. Therefore, it reacts with alcohols, phenols and oximes to the corresponding N,N-dimethylcarbamates, with thiols to thiolourethanes, with amines and hydroxylamines to substituted ureas, and with imidazoles and triazoles to carbamoylazoles.[10]
DMCC is less reactive and less selective to substrates with multiple nucleophilic centers than conventional acyl chlorides.
Unsaturated conjugated aldehydes such as crotonaldehyde (trans-but-2-enal) react with DMCC forming dienyl carbamates, which can be used as dienes in Diels–Alder reactions.[13]
Alkali metal carboxylates react with DMCC forming the corresponding dimethylamides. DMCC reacts with anhydrous sodium carbonate[14] or with excess dimethylamine to form tetramethylurea.[15]
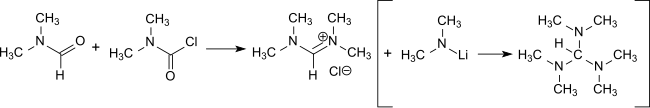
The reaction of DMCC with DMF forms tetramethylformamidinium chloride,[16] which is a major intermediate in the preparation of tris(dimethylamino)methane, a reagent for the introduction of enamine functions in conjunction with activated methylene groups[17] and the preparation of amidines.[18]
DMCC is a starting material for the insecticide class of the dimethyl carbamates which act as inhibitors of acetylcholinesterase, including dimetilane,[19] and the related compounds isolane, pirimicarb and triazamate.
The quaternary ammonium compounds neostigmine[20] finds pharmaceutical applications as acetylcholinesterase inhibitors. It is obtained from 3-(dimethylamino)phenol and DMCC and subsequent quaternization with methyl bromide or dimethyl sulfate[21]
and pyridostigmine, which is obtainable from 3-hydroxypyridine and DMCC and subsequent reaction with methyl bromide.[22]
DMCC is also used in the synthesis of the benzodiazepine camazepam.[23]
See also
References
- ^ Pohanish, R. P. (2011). Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens (6th ed.). Amsterdam: Elsevier. pp. 1045–1047. ISBN 978-1-4377-7869-4.
- ^ Michler, W.; Escherich, C. (1879). "Ueber mehrfach substituirte Harnstoffe" [On multiply-substituted ureas]. Berichte der Deutschen Chemischen Gesellschaft (in German). 12 (1): 1162–1164. doi:10.1002/cber.187901201303.
- ^ Slocombe, R. J.; Hardy, E. A.; Saunders, J. H.; Jenkins, R. L. (1950). "Phosgene derivatives. The preparation of isocyanates, carbamyl chlorides and cyanuric acid". Journal of the American Chemical Society. 72 (5): 1888–1891. doi:10.1002/ja01161a009 (inactive 31 January 2024).
{{cite journal}}: CS1 maint: DOI inactive as of January 2024 (link) - ^ Karimipour, G.; Kowkabi, S.; Naghiha, A. (2015). "New aminoporphyrins bearing urea derivative substituents: synthesis, characterization, antibacterial and antifungal activity". Brazilian Archives of Biology and Technology. 58 (3): 431–442. doi:10.1590/S1516-8913201500024.
- ^ Babad, H.; Zeiler, A. G. (1973). "Chemistry of phosgene". Chemical Reviews. 73 (1): 75–91. doi:10.1021/cr60281a005.
- ^ Saegusa, T.; Tsuda, T.; Isegawa, Y. (1971). "Carbamoyl chloride formation from chloramine and carbon monoxide". The Journal of Organic Chemistry. 36 (6): 858–860. doi:10.1021/jo00805a033.
- ^ Stare, M.; Laniewski, K.; Westermark, A.; Sjögren, M.; Tian, W. (2009). "Investigation on the formation and hydrolysis of N,N-dimethylcarbamoyl chloride (DMCC) in Vilsmeier reactions using GC/MS as the analytical detection method". Organic Process Research & Development. 13 (5): 857–862. doi:10.1021/op900018f.
- ^ Levin, D. (1997). "Potential toxicological concerns associated with carboxylic acid chlorination and other reactions". Organic Process Research & Development. 1 (2): 182. doi:10.1021/op970206t.
- ^ Queen, A. (1967). "Kinetics of the hydrolysis of acyl chlorides in pure water". Canadian Journal of Chemistry. 45 (14): 1619–1629. doi:10.1139/v67-264.
- ^ a b Kreutzberger, C. B.; Olofson, R. A. (2001). "Dimethylcarbamoyl chloride". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd319. ISBN 0-471-93623-5.
- ^ Jäger, P.; Rentzea, C. N.; Kieczka, H. (2014). "Carbamates and carbamoyl chloride". Ullmann's Fine Chemicals. Weinheim: Wiley-VCH. pp. 57–58. ISBN 978-3-527-33477-3.
- ^ "Dimethylcarbamoyl Chloride, CAS No. 79-44-7" (PDF). Report on Carcinogens (13th ed.). National Toxicology Program, Department of Health and Human Services. Retrieved 2016-09-25.
- ^ De Cusati, P. F.; Olofson, R. A. (1990). "A simple synthesis of 1-(1,3-butadienyl)carbonates and carbamates". Tetrahedron Letters. 31 (10): 1405–1408. doi:10.1016/S0040-4039(00)88817-6.
- ^ Lawson, J. K. Jr.; Croom, J. A. T. (1963). "Dimethylamides from alkali carboxylates and dimethylcarbamoyl chloride". The Journal of Organic Chemistry. 28 (1): 232–235. doi:10.1021/jo1036a513 (inactive 31 January 2024).
{{cite journal}}: CS1 maint: DOI inactive as of January 2024 (link) - ^ US 3597478, Weakly, M. L., "Preparation of tetramethylurea", issued 1971-08-03, assigned to Nipak Inc.
- ^ Arnold, Z. (1959). "The preparation of tetramethylformamidinium salts and their vinylogues". Collection of Czechoslovak Chemical Communications. 24 (3): 760–765. doi:10.1135/cccc19590760.
- ^ Meerwein, H.; Florian, W.; Schön, N.; Stopp, G. (1961). "Über Säureamidacetale, Harnstoffacetale und Lactamacetale" [On acid amide acetals, urea acetals and lactam acetals]. Justus Liebigs Annalen der Chemie (in German). 641 (1): 1–39. doi:10.1002/jlac.19616410102.
- ^ Bredereck, H.; Effenberger, F.; Brendle, Th. (1966). "Synthese und Reaktionen von Trisdimethylaminomethan" [Synthesis and reactions of tris(dimethylamino)methane] (PDF). Angewandte Chemie (in German). 78 (2): 147–148. Bibcode:1966AngCh..78..147B. doi:10.1002/ange.19660780212.
- ^ US 3452043, Grauer, T. & Urwyler, H., "Production of 1-N,N-dimethylcarbamoyl-5-methyl-3-N,N-dimethylcarbamoyloxypyrazole", issued 1969-06-24, assigned to J. R. Geigy AG
- ^ Aeschlimann, J. A.; Reinert, M. (1931). "Pharmacological action of some analogues of physostigmine". Journal of Pharmacology and Experimental Therapeutics. 43 (3): 413–444.
- ^ US 1905990, Aeschlimann, J. A., "Disubstituted carbamic acid esters of phenols containing a basic constituent", issued 1933-04-25, assigned to Hoffmann-La Roche Inc.
- ^ US 2572579, Urban, R., "Disubstituted carbamic acid esters of 3-hydroxy-1-alkyl-pyridinium salts", issued 1951-10-23, assigned to Hoffmann-La Roche Inc.
- ^ DE 2558015, "Verfahren zur Herstellung des 3-N,N-Dimethylcarbamoyl-oxy-1-methyl-5-phenyl-7-chlor-1,3-dihydro-2H-1,4-benzodiazepin-2-on (Process for the preparation of 3-N,N-dimethylcarbamoyloxy-1-methyl-5-phenyl-7-chloro-1,3-dihydro-2H-1,4-benzodiazepin-2-one)", issued 1976-09-16, assigned to Siphar SA
- CS1 German-language sources (de)
- CS1 maint: DOI inactive as of January 2024
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Acyl chlorides
- IARC Group 2A carcinogens