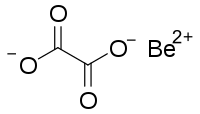
Beryllium oxalate
Jump to navigation
Jump to search
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C 2BeO 4 | |
| Molar mass | 97.03[1] |
| Appearance | Transparent crystals |
| Boiling point | 365.1 °C (689.2 °F; 638.2 K) |
| Soluble | |
| Hazards | |
| Flash point | 188.8[2] °C (371.8 °F; 461.9 K) |
| Related compounds | |
Related compounds
|
Calcium oxalate Sodium oxalate Magnesium oxalate Strontium oxalate Barium oxalate Iron(II) oxalate Iron(III) oxalate Lithium oxalate Praseodymium oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Beryllium oxalate is an inorganic compound, a salt of beryllium metal and oxalic acid with the chemical formula C
2BeO
4.[3] It forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is used to prepare ultra-pure beryllium oxide[4] by thermal decomposition.[5]
Synthesis
The action of oxalic acid on beryllium hydroxide:[6]
Chemical properties
Crystalline hydrates lose water when heated:
References
- ^ "BERYLLIUM OXALATE". chemicalbook.com. Retrieved 15 June 2021.
- ^ "beryllium,oxalate". chemsrc.com. Retrieved 15 June 2021.
- ^ Novoselova, Aleksandra Vasilʹevna; Bat︠s︡anova, Li︠u︡dmila Rafailovna (1969). Analytical Chemistry of Beryllium. Ann Arbor-Humphrey Science Publishers. p. 25. Retrieved 15 June 2021.
- ^ Dollimore, David; Konieczay, Julie L. (1998-09-07). "The thermal decomposition of beryllium oxalate and related materials". Thermochimica Acta. 318 (1–2): 155–163. doi:10.1016/S0040-6031(98)00340-2. Retrieved 15 June 2021.
- ^ Walsh, Kenneth A. (2009-01-01). Beryllium Chemistry and Processing. ASM International. p. 125. ISBN 978-0-87170-721-5. Retrieved 15 June 2021.
- ^ Moore, Raymond E. (1960). Purification of Beryllium Compounds: A Literature Survey. Oak Ridge National Laboratory. p. 6. Retrieved 15 June 2021.

![{\displaystyle {\mathsf {BeC_{2}O_{4}\cdot 3H_{2}O\ {\xrightarrow[{-2H_{2}O}]{100^{o}C}}\ BeC_{2}O_{4}\cdot H_{2}O\ {\xrightarrow[{-H_{2}O}]{220^{o}C}}\ BeC_{2}O_{4}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/26f1ccf7450ff492ba682cba56b28df05ac0b8c3)