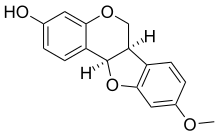
Medicarpin
Jump to navigation
Jump to search
| |
| Names | |
|---|---|
| IUPAC name
9-Methoxy-6a,11a-dihydro-6H-[1]benzofuro[3,2-c]chromen-3-ol
| |
| Other names
3-Hydroxy-9-methoxypterocarpan
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H14O4 | |
| Molar mass | 270.27 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Medicarpin is a pterocarpan, a derivative of isoflavonoids.
Natural occurrences
Medicarpin is found in Medicago truncatula and Swartzia madagascariensis. It can also be found in Maackia amurensis cell cultures.[1]
The root nodule formation by Sinorhizobium meliloti[2] is apparently dependent on the flavonoids pathway.[3]
Metabolism
Pterocarpin synthase has 3 substrates : medicarpin, NADP+ and H2O, and 3 products : vestitone, NADPH and H+.[4]

References
- ^ Isoflavonoid production by callus cultures of Maackia amurensis. S.A Fedoreyev, T.V Pokushalov, M.V Veselova, L.I Glebko, N.I Kulesh, T.I Muzarok, L.D Seletskaya, V.P Bulgakov and Yu.N Zhuravlev, Fitoterapia, 1 August 2000, Volume 71, Issue 4, Pages 365–372, doi:10.1016/S0367-326X(00)00129-5
- ^ Dakora FD, Joseph CM, Phillips DA (1993). "Alfalfa (Medicago sativa L.) Root Exudates Contain Isoflavonoids in the Presence of Rhizobium meliloti". Plant Physiol. 101 (3): 819–824. doi:10.1104/pp.101.3.819. PMC 158695. PMID 12231731.
- ^ Wasson, A. P. (2006). "Silencing the Flavonoid Pathway in Medicago truncatula Inhibits Root Nodule Formation and Prevents Auxin Transport Regulation by Rhizobia". The Plant Cell Online. 18 (7): 1617–1629. doi:10.1105/tpc.105.038232. PMC 1488924. PMID 16751348.
- ^ Lining Guo, Richard A. Dixon and Nancy L. Paival (1994). "Conversion of Vestitone to Medicarpin in Alfalfa (Medicago sativa L.) Is Catalyzed by Two Independent Enzymes. Identification, Purification, and Characterization of Vestitone Reductase and 7,2'-Dihydroxy-4'-MethoxyIsoflavanol Dehydratase". Journal of Biological Chemistry. 269 (35): 22372–22378. doi:10.1016/S0021-9258(17)31799-4. PMID 8071365.
Categories:
- Articles with changed EBI identifier
- Articles with changed ChemSpider identifier
- Articles with changed KEGG identifier
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Articles with short description
- Short description is different from Wikidata
- Pterocarpans
- Methoxy compounds
- All stub articles
- Aromatic compound stubs