Magnesium formate
Jump to navigation
Jump to search
This article needs additional citations for verification. (November 2015) |
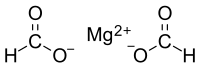
| |
| Names | |
|---|---|
| IUPAC name
Magnesium diformate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.341 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mg(HCO2)2 | |
| 14 g/100g at 0 °C 14.4 g/100g at 20 °C | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium formate is a magnesium salt of formic acid. It is an inorganic compound. It consists of a magnesium cation and formate anion. It can be prepared by reacting magnesium oxide with formic acid. The dihydrate is formed when crystallizing from the solution. The dihydrate dehydrates at 105 °C to form anhydrate, then decomposes at 500 °C to produce magnesium oxide.[1] Magnesium formate can be used for organic syntheses.[2]
References
- ^ D. Dollimore, J.P. Gupta, D.V. Nowell (May 1979). "The thermal decomposition of metal formates. II. Solid state thermal decomposition studies on magnesium formate dihydrate". Thermochimica Acta. 30 (1–2): 339–350. doi:10.1016/0040-6031(79)85069-8. ISSN 0040-6031. Archived from the original on 2018-06-30. Retrieved 2018-08-09.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brückner, Reinhard; Zettlmeier, Wolfgang (2015). Reaktionsmechanismen organische Reaktionen, Stereochemie, moderne Synthesemethoden (in German). Berlin. p. 316. ISBN 978-3-662-45683-5. OCLC 901537772.
{{cite book}}: CS1 maint: location missing publisher (link)
Categories:
- CS1 maint: multiple names: authors list
- CS1 maint: location missing publisher
- CS1 German-language sources (de)
- Articles needing additional references from November 2015
- All articles needing additional references
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Formates
- Magnesium compounds
- All stub articles
- Inorganic compound stubs