MES (buffer)
| |
| Names | |
|---|---|
| IUPAC names
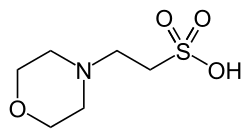
2-morpholin-4-ylethanesulfonic acid
Zwitterion: 2-morpholin-4-ium-4-ylethanesulfonate | |
| Other names
2-(N-morpholino)ethanesulfonic acid
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.022.394 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H13NO4S | |
| Molar mass | 195.2 g/mol |
| Acidity (pKa) | 6.15[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
MES (2-(N-morpholino)ethanesulfonic acid) is a chemical compound that contains a morpholine ring. It has a molecular weight of 195.2 g/mol and the chemical formula is C6H13NO4S. Synonyms include: 2-morpholinoethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; 2-(N-morpholino)ethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; MES; MES hydrate; and morpholine-4-ethanesulfonic acid hydrate. MOPS is a similar pH buffering compound which contains a propanesulfonic moiety instead of an ethanesulfonic one.
Applications
MES is used as a buffering agent in biology and biochemistry. It has pKa value of 6.15 at 20 °C. The pH (and pKa at ionic strength I≠0) of the buffer solution changes with concentration and temperature, and this effect may be predicted using online calculators.[2] MES is highly soluble in water. The melting point is approx. 300 °C.
MES was developed as one of Good's buffers in the 1960s. These buffers were developed with the following criteria in mind: midrange pKa, maximum water solubility and minimum solubility in all other solvents, minimal salt effects, minimal change in pKa with temperature, chemically and enzymatically stable, minimal absorption in visible or UV spectral range and reasonably easily synthesized.[1] MES is also useful as a non-coordinating buffer in chemistry involving metal ions, as many common buffers (e.g. phosphate and acetate) readily form coordination complexes. MES only weakly binds Ca, Mg, Mn, and it has negligible binding with Cu(II).[1][3]
Effect of impurities
Commercial preparations of MES (and other sulfonylethyl buffers like BES, CHES, and PIPES) can contain a contaminant oligo(vinylsulfonic acid) (OVS), which is a polyanionic mimic of RNA, and can be a potent (pM) inhibitor of RNA binding proteins and enzymes.[4]
Safety
Contact with this buffer is hazardous;[5] skin or eye exposure should be cleaned well with water and medical aid should be sought in the case of eye exposure, swallowing, or inhalation of dust. It also emits toxic fumes upon combustion, including carbon monoxide, nitrogen oxide, and sulfur oxides.
See also
References
- ^ a b c Good, Norman E.; Winget, G. Douglas; Winter, Wilhelmina; Connolly, Thomas N.; Izawa, Seikichi; Singh, Raizada M. M. (1966). "Hydrogen Ion Buffers for Biological Research". Biochemistry. 5 (2): 467–77. doi:10.1021/bi00866a011. PMID 5942950.
- ^ "Biological buffers". REACH Devices.
- ^ Kandegedara, A.; Rorabacher, D. B. (1999). "Noncomplexing Tertiary Amines as "Better" Buffers Covering the Range of pH 3−11. Temperature Dependence of Their Acid Dissociation Constants". Anal. Chem. 71 (15): 3140–3144. doi:10.1021/ac9902594. PMID 21662904.
- ^ Smith, Bryan D.; Soellner, Matthew B.; Raines, Ronald T. (2003). "Potent Inhibition of Ribonuclease A by Oligo(vinylsulfonic Acid)". Journal of Biological Chemistry. 278 (23). Elsevier BV: 20934–20938. doi:10.1074/jbc.m301852200. ISSN 0021-9258.
- ^ "Material Safety Data Sheet". Archived from the original on 2018-09-20. Retrieved 2012-09-10.
External links
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Articles without KEGG source
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Buffer solutions
- Sulfonic acids
- 4-Morpholinyl compounds