Immunoassay
| Immunoassay | |
|---|---|
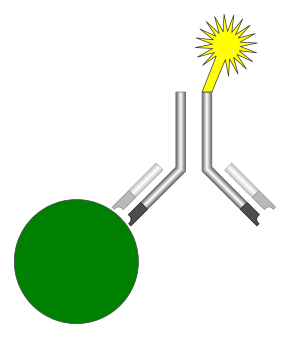 Illustration of the basic components of an immunoassay, which includes an analyte (green), an antibody (black), and a detectable label (yellow) | |
| Purpose | Test that measures the presence of a macromolecule |
| MeSH | D007118 |
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody or an antigen . The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.[1]
Immunoassays come in many different formats and variations. Immunoassays may be run in multiple steps with reagents being added and washed away or separated at different points in the assay. Multi-step assays are often called separation immunoassays or heterogeneous immunoassays. Some immunoassays can be carried out simply by mixing the reagents and samples and making a physical measurement. Such assays are called homogeneous immunoassays.[2][3]
The use of a calibrator is often employed in immunoassays, calibrators are solutions that are known to contain the analyte in question, and the concentration of that analyte is generally known. Comparison of an assay's response to a real sample against the assay's response produced by the calibrators makes it possible to interpret the signal strength in terms of the presence or concentration of analyte in the sample.[4][5]
Principle
Immunoassays rely on the ability of an antibody to recognize and bind a specific macromolecule in what might be a complex mixture of macromolecules. In immunology the particular macromolecule bound by an antibody is referred to as an antigen and the area on an antigen to which the antibody binds is called an epitope.In some cases, an immunoassay may use an antigen to detect for the presence of antibodies, which recognize that antigen, in a solution. In other words, in some immunoassays, the analyte may be an antibody rather than an antigen.In addition to the binding of an antibody to its antigen, the other key feature of all immunoassays is a means to produce a measurable signal in response to the binding. Most immunoassays involve chemically linking antibodies or antigens with some kind of detectable label. A large number of labels exist in modern immunoassays, and they allow for detection through different means. Many labels are detectable because they either emit radiation, produce a color change in a solution, fluoresce under light, or can be induced to emit light.[6][7][2]
Use

Clinical tests
A wide range of medical tests are immunoassays, called immunodiagnostics in this context. Many home pregnancy tests are immunoassays, which detect the pregnancy marker human chorionic gonadotropin.[8][9]
Other clinical immunoassays include tests that measure levels of CK-MB to assess heart disease, insulin to assess hypoglycemia, prostate-specific antigen to detect prostate cancer, as well as breast cancer, and some are also used for the detection and/or quantitative measurement of some pharmaceutical compounds .[10][11]
Anti-doping analysis (sports)
Immunoassays are used in sports anti-doping laboratories to test athletes' blood samples for prohibited recombinant human growth hormone (rhGH, rGH, hGH, GH).[12]
Classifications and formats

Immunoassays can be run in a number of different formats. Generally, an immunoassay will fall into one of several categories depending on how it is run.[13]
Competitive, homogeneous immunoassays
In a competitive, homogeneous immunoassay, unlabelled analyte in a sample competes with labeled analyte to bind an antibody. The amount of labelled, unbound analyte is then measured.[14].

- The fluorescence polarization immunoassay (FPIA) measures the fluorescence polarization signal after incubation, without separating bound and free labels. Free labeled analyte analog molecules are added to the sample, and their Brownian motion differs when bound to a large antibody (Ab) versus free in solution. The analyte competes for binding to the Ab, and if the labeled analyte binds to the Ab, a signal is produced. The signal intensity is inversely proportional to the analyte concentration.[15]
- In the enzyme multiplied immunoassay technique (EMIT), free analyte analog molecules labeled with an enzyme (e.g., glucose-6-phosphate dehydrogenase enzyme) compete with the analyte being tested. The active enzyme reduces NAD (no signal) to NADH (which absorbs at 340 nm), so absorbance is monitored at 340 nm. When the labeled analyte binds to the Ab, the enzyme becomes inactive, and a signal is generated by the free label. The signal intensity is directly proportional to the analyte concentration.[15]
- The luminescent oxygen channeling immunoassay (LOCI) generates singlet oxygen species in microbeads coupled to the analyte, and when the analyte binds to the respective Ab molecule, coupled to another kind of bead, the analyte reacts with singlet oxygen, generating chemiluminescence signals proportional to the concentration of the analyte-Ab complex.[15]
- In the kinetic interaction of microparticle in solution (KIMS) and particle enhanced turbidimetric inhibition immunoassay (PETINIA), free antibodies bind to drug microparticle conjugates to form aggregates that absorb in the visible range in the absence of the analyte. In the presence of the analyte, the Ab binds to the free analyte, preventing microparticle aggregation and causing a reduction in absorbance. The signal is inversely proportional to the analyte concentration.[15]
- The cloned enzyme donor immunoassay (CEDIA) involves genetically engineering an enzyme (e.g., beta-galactosidase) into two inactive fragments: a small enzyme donor (ED) conjugated with the drug analog, and a larger enzyme acceptor (EA). When the two fragments associate, the full enzyme converts a substrate into a cleaved colored product. If drug analyte molecules are present, they compete with the ED-labeled drug in solution for the limited Ab sites. Free ED-labeled drug analog will bind to EA, generating a colorimetric signal directly proportional to the amount of analyte.[15][16]

Competitive, heterogeneous immunoassays
As in a competitive, homogeneous immunoassay, unlabelled analyte in a sample competes with labelled analyte to bind an antibody. In the heterogeneous assays, the labelled, unbound analyte is separated or washed away, and the remaining labelled, bound analyte is measured.[17][18]
One-site, noncompetitive immunoassays
The unknown analyte in the sample binds with labelled antibodies. The unbound, labelled antibodies are washed away, and the bound, labelled antibodies are measured. The intensity of the signal is directly proportional to the amount of unknown analyte.[19][20]
Two-site, noncompetitive immunoassays
The analyte in the unknown sample is bound to the antibody site, then the labelled antibody is bound to the analyte. The amount of labelled antibody on the site is then measured, it will be directly proportional to the concentration of the analyte because the labelled antibody will not bind if the analyte is not present in the unknown sample, this type of immunoassay is also known as a sandwich assay as the analyte is "sandwiched" between two antibodies.[21][22][23]
Labels

Immunoassays employ a variety of different labels to allow for detection of antibodies and antigens; labels are typically chemically linked or conjugated to the desired antibody or antigen.[19]
Enzymes
Possibly one of the most popular labels to use in immunoassays is enzymes. Immunoassays which employ enzymes are referred to as enzyme immunoassays (EIAs), of which enzyme-linked immunosorbent assays (ELISAs) and enzyme multiplied immunoassay technique (EMIT) are the most common types.[24][25]
Enzymes used in ELISAs include horseradish peroxidase (HRP), alkaline phosphatase (AP) or glucose oxidase. These enzymes allow for detection often because they produce an observable color change in the presence of certain reagents. In some cases these enzymes are exposed to reagents which cause them to produce light or chemiluminescence.[26]
Radioactive isotopes
Radioactive isotopes can be incorporated into immunoassay reagents to produce a radioimmunoassay (RIA). Radioactivity emitted by bound antibody-antigen complexes can be easily detected using conventional methods.[27][28]
RIAs were some of the earliest immunoassays developed, but have fallen out of favor largely due to the difficulty and potential dangers presented by working with radioactivity.[29][30]
DNA reporters
A newer approach to immunoassays involves combining real-time quantitative polymerase chain reaction (RT qPCR) and traditional immunoassay techniques. Called real-time immunoquantitative PCR (iqPCR) the label used in these assays is a DNA probe.[31][32]
Fluorogenic reporters
Fluorogenic reporters like phycoerythrin are used in a number of modern immunoassays.[33] Protein microarrays are a type of immunoassay that often employ fluorogenic reporters.[34]
Electrochemiluminescent tags
Some labels work via electrochemiluminescence (ECL), in which the label emits detectable light in response to electric current.[35][36]
Label-free immunoassays
While some kind of label is generally employed in immunoassays, there are certain kinds of assays which do not rely on labels, but instead employ detection methods that do not require the modification or labeling the components of the assay. Surface plasmon resonance is an example of technique that can detect binding between an unlabeled antibody and antigens.[37] Another demonstrated labeless immunoassay involves measuring the change in resistance on an electrode as antigens bind to it.[38]
History

Rosalyn Sussman Yalow and Solomon Berson are credited with the development of the first immunoassays in the 1950s. Yalow accepted the Nobel Prize for her work in immunoassays in 1977, becoming the second American woman to have won the award.[39]
Immunoassays became considerably simpler to perform and more popular when techniques for chemically linked enzymes to antibodies were demonstrated in the late 1960s.[40]
In 1983, Professor Anthony Campbell[41] at Cardiff University replaced radioactive iodine used in immunoassay with an acridinium ester that makes its own light: chemiluminescence. This type of immunoassay is now used in around 100 million clinical tests every year worldwide, enabling clinicians to measure a wide range of proteins, pathogens and other molecules in blood samples.[42]
By 2012, the commercial immunoassay industry earned US$17,000,000,000 and was thought to have prospects of slow annual growth in the 2 to 3 percent range.[43]
Research
The photoacoustic immunoassay measures low-frequency acoustic signals generated by metal nanoparticle tags. Illuminated by a modulated light at a plasmon resonance wavelength, the nanoparticles generate strong acoustic signal, which can be measured using a microphone.[44] The photoacoustic immunoassay can be applied to lateral flow tests, which use colloidal nanoparticles.[45]
See also
References
- ↑ Yetisen A. K. (2013). "Paper-based microfluidic point-of-care diagnostic devices". Lab on a Chip. 13 (12): 2210–2251. doi:10.1039/C3LC50169H. PMID 23652632.
- ↑ 2.0 2.1 Cox, Karen L.; Devanarayan, Viswanath; Kriauciunas, Aidas; Manetta, Joseph; Montrose, Chahrzad; Sittampalam, Sitta (2004). "Immunoassay Methods". Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences. Archived from the original on 2023-01-19. Retrieved 2023-10-20.
- ↑ Hage, David S. (1 June 1999). "Immunoassays". Analytical Chemistry. 71 (12): 294–304. doi:10.1021/a1999901+. ISSN 0003-2700. Retrieved 22 October 2023.
- ↑ Rej, R.; Drake, P. (1991). "The nature of calibrators in immunoassays: are they commutable with test samples? Must they be?". Scandinavian Journal of Clinical and Laboratory Investigation. Supplementum. 205: 47–54. doi:10.3109/00365519109104601. ISSN 0085-591X. Archived from the original on 2023-10-26. Retrieved 2023-10-25.
- ↑ "Standards, Calibrators, and Controls in Immunoassays - DCN Dx". dcndx. 27 January 2020. Archived from the original on 29 October 2023. Retrieved 28 October 2023.
- ↑ Alice Lee, N.; Kennedy, Ivan R. (1 January 2007). "Chapter 5 - Immunoassays". Food Toxicants Analysis. Elsevier. pp. 91–145. ISBN 978-0-444-52843-8. Archived from the original on 26 October 2023. Retrieved 23 October 2023.
- ↑ Kohl, Thomas O.; Ascoli, Carl A. (5 July 2017). "Immunoassays". Cold Spring Harbor Protocols. 2017 (7): pdb.top093690. doi:10.1101/pdb.top093690. ISSN 1559-6095. Archived from the original on 21 October 2021. Retrieved 26 October 2023.
- ↑ "ELISA for Home Pregnancy Test". Archived from the original on 21 September 2017. Retrieved 11 December 2012.
- ↑ Butler, SA (2001). "Detection of early pregnancy forms of human chorionic gonadotropin by home pregnancy test devices". Clinical Chemistry. 47 (12): 2131–6. doi:10.1093/clinchem/47.12.2131. PMID 11719477.
- ↑ Twyman, SA (2001). "Immunoassays, applications: Clinica" (PDF). Encyclopedia of Analytical Science. 4: 317–324. Archived from the original (PDF) on 24 March 2012. Retrieved 11 December 2012.
- ↑ "Immunoassay". www.cancer.gov. 2011. Archived from the original on 25 October 2022. Retrieved 20 October 2023.
- ↑ Tsivou M; Kioukia-Fougia N; Lyris E; Aggelis Y; Fragkaki A; Kiousi X; Simitsek Ph; Dimopoulou H; Leontiou I-P; Stamou M; Spyridaki M-H; Georgakopoulos C (2006). "An overview of the doping control analysis during the Olympic Games of 2004 in Athens, Greece". Analytica Chimica Acta. 555: 1–13. doi:10.1016/j.aca.2005.08.068.
- ↑ Goldys, Ewa (2009-08-24). Fluorescence Applications in Biotechnology and Life Sciences. Wiley-Blackwell. p. 311. ISBN 978-0470083703. Archived from the original on 2023-10-20. Retrieved 11 December 2012. License: "CC BY 4.0"
- ↑ Hepojoki, Satu; Rusanen, Juuso; Hepojoki, Jussi; Nurmi, Visa; Vaheri, Antti; Lundkvist, Åke; Hedman, Klaus; Vapalahti, Olli (July 2015). "Competitive Homogeneous Immunoassay for Rapid Serodiagnosis of Hantavirus Disease". Journal of Clinical Microbiology. 53 (7): 2292–2297. doi:10.1128/JCM.00663-15. ISSN 1098-660X. Archived from the original on 2022-06-30. Retrieved 2023-10-23.
- ↑ Burtis, Carl A., ed. (2012). Tietz Textbook of Clinical Biochemistry and Molecular diagnostics. USA: Elsevier Saunders. p. 393. ISBN 9781416061649.
- ↑ "Methodology: Immunoassays | myADLM.org". www.aacc.org. Archived from the original on 17 August 2022. Retrieved 21 October 2023.
- ↑ Murphy, Brian M.; He, Xinya; Dandy, David; Henry, Charles S. (15 January 2008). "Competitive Immunoassays for Simultaneous Detection of Metabolites and Proteins Using Micromosaic Patterning". Analytical chemistry. 80 (2): 444–450. doi:10.1021/ac7019046. ISSN 0003-2700. Archived from the original on 22 October 2023. Retrieved 21 October 2023.
- ↑ 19.0 19.1 Lynch, Andrea (3 October 2022). "Types Of Immunoassay - And When To Use Them". Quanterix. Archived from the original on 20 March 2023. Retrieved 21 October 2023.
- ↑ Zhang, Xiaojin; Xia, Fan (2018). "Introduction". Biosensors Based on Sandwich Assays. Springer. pp. 1–13. ISBN 978-981-10-7835-4. Archived from the original on 8 June 2018. Retrieved 27 October 2023.
- ↑ Colon, Paula Jenkins; Greene, Dina N (1 May 2018). "Biotin Interference in Clinical Immunoassays". The Journal of Applied Laboratory Medicine. 2 (6): 941–951. doi:10.1373/jalm.2017.024257. Archived from the original on 24 October 2022. Retrieved 21 October 2023.
- ↑ Tanaka, Koichiro; Kohno, Takeyuki; Hashida, Seiichi; Lshikawa, Eiji (January 1990). "Novel and sensitive noncompetitive (two‐site) enzyme immunoassay for haptens with amino groups". Journal of Clinical Laboratory Analysis. 4 (3): 208–212. doi:10.1002/jcla.1860040312. ISSN 0887-8013. Archived from the original on 2023-10-26. Retrieved 2023-10-24.
- ↑ Darwish, Ibrahim A. (September 2006). "Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances". International journal of biomedical science: IJBS. 2 (3): 217–235. ISSN 1550-9702. Archived from the original on 2 April 2023. Retrieved 31 October 2023.
- ↑ Alhajj, Mandy; Zubair, Muhammad; Farhana, Aisha (2023). "Enzyme Linked Immunosorbent Assay". StatPearls. StatPearls Publishing. Archived from the original on 2021-09-25. Retrieved 2023-10-22.
- ↑ Ullman, Edwin F. (2013). "Homogeneous Immunoassay". In Wild, David (ed.). The Immunoassay Handbook (4th ed.). pp. 76–77. ISBN 9780080970370. Archived from the original on 2023-10-14. Retrieved 2023-10-22.
- ↑ Khatkhatay, M. I.; Desai, M. (August 1999). "A comparison of performances of four enzymes used in ELISA with special reference to beta-lactamase". Journal of Immunoassay. 20 (3): 151–183. doi:10.1080/01971529909349349. ISSN 0197-1522. Archived from the original on 22 October 2023. Retrieved 22 October 2023.
- ↑ Shan, Guomin; Huang, Wei; Gee, Shirley J.; Buchholz, Bruce A.; Vogel, John S.; Hammock, Bruce D. (14 March 2000). "Isotope-labeled immunoassays without radiation waste". Proceedings of the National Academy of Sciences of the United States of America. 97 (6): 2445–2449. ISSN 0027-8424. Archived from the original on 26 October 2023. Retrieved 23 October 2023.
- ↑ Chu, F. S. (1 January 2003). "IMMUNOASSAYS | Radioimmunoassay and Enzyme Immunoassay". Encyclopedia of Food Sciences and Nutrition (Second Edition). Academic Press. pp. 3248–3255. ISBN 978-0-12-227055-0. Archived from the original on 29 October 2023. Retrieved 26 October 2023.
- ↑ Susan J. Landers (3 April 2006). "ELISA test marks 35 years of answering medical questions". American Medical News. Archived from the original on 10 November 2011. Retrieved 9 December 2012.
- ↑ "Rosalyn Sussman Yalow". America.gov. April 27, 2008. Archived from the original on October 20, 2012. Retrieved June 26, 2010.
- ↑ Rajkovic; El-Moualij (2006). "Immunoquantitative real-time PCR for detection and quantification of Staphylococcus aureus enterotoxin B in foods". Applied and Environmental Microbiology. 72 (10): 6593–9. Bibcode:2006ApEnM..72.6593R. doi:10.1128/AEM.03068-05. PMC 1610299. PMID 17021210.
- ↑ Gofflot; El (2004). "Immuno-quantitative polymerase chain reaction for detection and quantitation of prion protein". Journal of Immunoassay and Immunochemistry. 25 (3): 241–58. doi:10.1081/ias-200028044. PMID 15461386. S2CID 37548553.
- ↑ "Luminex xMAP Technology". Millipore Corporation. Retrieved 13 December 2012.
- ↑ Chatterjee; Sitaraman (2008). "Protein microarray on-demand: a novel protein microarray system". PLOS ONE. 3 (9): e3265. Bibcode:2008PLoSO...3.3265C. doi:10.1371/journal.pone.0003265. PMC 2533396. PMID 18813342.
- ↑ Zanut, A.; Fiorani, A.; Canola, S.; Saito, T.; Ziebart, N.; Rapino, S.; Rebeccani, S.; Barbon, A.; Irie, T.; Josel, H.; Negri, F.; Marcaccio, M.; Windfuhr, M.; Imai, K.; Valenti, G.; Paolucci, F. (2020). "Insights into the mechanism of coreactant electrochemiluminescence facilitating enhanced bioanalytical performance". Nat. Commun. 11 (1): 2668. Bibcode:2020NatCo..11.2668Z. doi:10.1038/s41467-020-16476-2. PMC 7260178. PMID 32472057.
- ↑ Forster RJ, Bertoncello P, Keyes TE (2009). "Electrogenerated Chemiluminescence". Annual Review of Analytical Chemistry. 2: 359–85. Bibcode:2009ARAC....2..359F. doi:10.1146/annurev-anchem-060908-155305. PMID 20636067.
- ↑ J. B. González-Díaz; et al. (2008). "Plasmonic Au/Co/Au nanosandwiches with Enhanced Magneto-Optical Activity". Small. 4 (2): 202–5. doi:10.1002/smll.200700594. hdl:10261/17402. PMID 18196506. S2CID 206490102.
- ↑ Georgios Tsekenis (2008). "Label-free immunosensor assay for myelin basic protein based upon an ac impedance protocol". Analytical Chemistry. 80 (6): 2058–62. doi:10.1021/ac702070e. PMID 18260654.
- ↑ Rall JE. Solomon A. Berson. In "Biographical Memoirs". National Academy of Sciences 1990;59:54-71. ISBN 0-309-04198-8. Fulltext Archived 2007-03-13 at the Wayback Machine.
- ↑ Lequin R (2005). "Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA)". Clin. Chem. 51 (12): 2415–8. doi:10.1373/clinchem.2005.051532. PMID 16179424.
- ↑ Prof Anthony Campbell - MA PhD, Cardiff University, archived from the original on 18 April 2012, retrieved 29 December 2012
- ↑ "NPS Focus", Rainbow makers, Royal Society of Chemistry (RSC), 2003, archived from the original on 4 March 2016, retrieved 29 December 2012
- ↑ Carlson, Bruce (15 February 2014). "Seizing Immunoassay Opportunities". Gen. Eng. Biotechnol. News. Vol. 34, no. 4. pp. 12–13. Archived from the original on 15 March 2014. Retrieved 29 September 2023.
- ↑ Zhao Y, Cao M, McClelland JF, Lu M (2016). "A photoacoustic immunoassay for biomarker detection". Biosensors and Bioelectronics. 85: 261–66. doi:10.1016/j.bios.2016.05.028. PMID 27183276.
- ↑ Zhao Y, Huang Y, Zhao X, McClelland JF, Lu M (2016). "Nanoparticle-based photoacoustic analysis for highly sensitive lateral flow assays". Nanoscale. 8 (46): 19204–19210. doi:10.1039/C6NR05312B. PMID 27834971.
Further reading
- "The Immunoassay Handbook", 3rd Edition, David Wild, Ed., Elsevier,2008
External links
- Immunoassay at the US National Library of Medicine Medical Subject Headings (MeSH)
- Chapter 5 and 6 in the book "Bioanalytical Chemistry" by Susan R. Mikkelsen