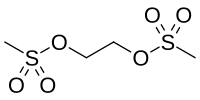
Ethane dimethanesulfonate
Jump to navigation
Jump to search
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethane-1,2-diyl di(methanesulfonate) | |
| Other names
Ethane 1,2-dimethane sulfonate
Ethane dimethanesulphonate | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | EDS |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O6S2 | |
| Molar mass | 218.24 g·mol−1 |
| Density | 1.65 g/cm3 |
| Melting point | 35–36 °C (95–97 °F; 308–309 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethane dimethanesulfonate (EDS) is an organic compound with formula (CH2OSO2CH3)2. It can be regarded as the esterification product of one glycol and two Methanesulfonic acids. EDS can eliminate all adult Leydig cells in testis of adult male rats, after which Leydig cells will regenerate from stem cells.[1]
See also
References
- ^ Risbridger, GP; Davies, A (June 1994). "Isolation of rat Leydig cells and precursor forms after administration of ethane dimethane sulfonate". The American Journal of Physiology. 266 (6 Pt 1): E975-9. doi:10.1152/ajpendo.1994.266.6.E975. PMID 8023929.