Curium(IV) oxide
Jump to navigation
Jump to search
| |
| Names | |
|---|---|
| Other names
Curium dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.453 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| CmO2 | |
| Molar mass | 279 g·mol−1 |
| Appearance | black crystals |
| insoluble | |
| Related compounds | |
Other cations
|
Americium(IV) oxide Berkelium(IV) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Curium(IV) oxide is an inorganic chemical compound of curium and oxygen with the chemical formula CmO2. Since all isotopes of curium are man-made, the compound does not occur in nature.
Synthesis
- Curium(IV) oxide can be prepared directly from the elements. Metallic curium is annealed in air or in an oxygen atmosphere:[1]
- Cm + O2 → CmO2
- Curium(III) hydroxide and curium(III) oxalate are also usually used for this purpose:
- Cm(OH)4 → CmO2 + 2H2O
- Cm(C2O4)2 → CmO2 + 2CO2 + 2CO
- Another way is the reaction of curium(III) oxide in an oxygen atmosphere at 650 °C:[2]
- 2Cm2O3 + O2 → 4CmO2
Physical properties
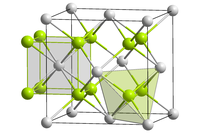
Curium(IV) oxide forms black crystals.[3] Insoluble in water. The compound crystals are of the cubic crystal system, the fluorite structure in the space group Fm3m.
Chemical properties
The compound reacts with mineral acids to form solutions of curium(III) salts.[4]
Uses
The compound is used for the manufacturing of isotopic current sources, also as targets for the synthesis of transcurium elements.
References
- ^ Asprey, L. B.; Ellinger, F. H.; Fried, S.; Zachariasen, W. H. (March 1955). "EVIDENCE FOR QUADRIVALENT CURIUM: X-RAY DATA ON CURIUM OXIDES1". Journal of the American Chemical Society. 77 (6): 1707–1708. doi:10.1021/ja01611a108. ISSN 0002-7863. Retrieved 29 June 2023.
- ^ Noé, M.; Fuger, J. (1 May 1971). "Self-radiation effects on the lattice parameter of 244CmO2". Inorganic and Nuclear Chemistry Letters. 7 (5): 421–430. doi:10.1016/0020-1650(71)80177-0. ISSN 0020-1650. Retrieved 29 June 2023.
- ^ Konings, R. J. M. (1 October 2001). "Thermochemical and thermophysical properties of curium and its oxides". Journal of Nuclear Materials. 298 (3): 255–268. Bibcode:2001JNuM..298..255K. doi:10.1016/S0022-3115(01)00652-3. ISSN 0022-3115. Retrieved 29 June 2023.
- ^ Lumetta, Gregg J.; Thompson, Major C.; Penneman, Robert A.; Eller, P. Gary (2006). "Curium". The Chemistry of the Actinide and Transactinide Elements. Springer Netherlands: 1397–1443. doi:10.1007/1-4020-3598-5_9. ISBN 978-1-4020-3555-5. Retrieved 29 June 2023.
Categories:
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Articles without UNII source
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Curium compounds
- Oxides