Bromotoluene
Bromotoluenes are aryl bromides based on toluene in which at least one aromatic hydrogen atom is replaced with a bromine atom. They have the general formula C7H8–nBrn, where n = 1–5 is the number of bromine atoms.
Monobromotoluene
Monobromotoluenes are bromotoluenes containing one bromine atom. There are three isomers, each with the formula C7H7Br.
Properties
The isomers differ in the location of the bromine, but have the same chemical formula.
| Monobromotoluene isomers[1][2][3] | ||||
|---|---|---|---|---|
| Common name | o-bromotoluene | m-bromotoluene | p-bromotoluene | |
| Structure | 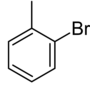
|
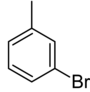
|

| |
| Systematic name | 1-bromo-2-methylbenzene | 1-bromo-3-methylbenzene | 1-bromo-4-methylbenzene | |
| Molecular formula | C7H7Br (C6H4BrCH3) | |||
| Molar mass | 171.03 g/mol | |||
| Appearance | colorless liquid | colorless liquid | white crystalline solid | |
| CAS number | [95-46-5] | [591-17-3] | [106-38-7] | |
| Properties | ||||
| Density and phase | 1.431 g/ml, liquid | 1.4099 g/ml, liquid | 1.3995 g/ml, solid | |
| Solubility in water | practically insoluble | |||
| Other solubilities | very soluble in ethanol, ether, benzene, carbon tetrachloride, acetone, chloroform | |||
| Melting point | -27.8 °C (-18.0 °F; -409.63 K) | -39.8 °C (-39.6 °F; -388.03 K) | 28.5 °C (83.3 °F; 301.7 K) | |
| Boiling point | 181.7 °C (359.1 °F; 454.9 K) | 183.7 °C (362.7 °F; 456.9 K) | 184.5 °C (364.1 °F; 457.7 K) | |
Benzyl bromide is an isomer, which has a bromine substituted for one of the hydrogens of toluene's methyl group, and it is sometimes named α-bromotoluene.
Preparation
A laboratory route to p-bromotoluene proceeds from p-toluidine, which is diazotiized followed by treatment with copper(I) bromide.[4]
Uses
Bromotoluenes are precursors to many organic building blocks. For example, the methyl group may be oxidized using potassium permanganate to form the corresponding bromobenzoic acid.[5] The methyl group may also be partially oxidized to form bromobenzaldehyde.[6]
See also
References
- ^ "2-Bromotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ^ "3-Bromotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ^ "4-Bromotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ^ Bigelow, L. A. (1925). "p-BROMOTOLUENE". Organic Syntheses. 5: 21. doi:10.15227/orgsyn.005.0021.
- ^ Bigelow, L. A. (1922). "A Study of Side-Chain Oxidations with Potassium Permanganate. Ii". Journal of the American Chemical Society. 44 (9): 2010–2019. doi:10.1021/ja01430a020.
- ^ Coleman, G. H.; Honeywell, G. E. (1937). "p-BROMOBENZALDEHYDE". Organic Syntheses. 17: 20. doi:10.15227/orgsyn.017.0020.