Bis(benzonitrile)palladium dichloride
Jump to navigation
Jump to search
| |

| |
| Names | |
|---|---|
| Other names
palladium dichloride sis(benzonitrile), bis(benzonitrile)dichloropalladium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.034.608 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C14H10Cl2N2Pd | |
| Molar mass | 383.57 g·mol−1 |
| Appearance | yellow-brown |
| Melting point | 129–130 °C (264–266 °F; 402–403 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H301, H311, H330 | |
| P260, P261, P264, P270, P271, P273, P280, P284, P301+P310, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P310, P311, P312, P320, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
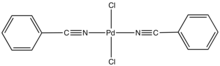
Bis(benzonitrile)palladium dichloride is the coordination complex with the formula PdCl2(NCC6H5)2. It is the adduct of two benzonitrile (PhCN) ligands with palladium(II) chloride. It is a yellow-brown solid that is soluble in organic solvents. The compound is a reagent and a precatalyst for reactions that require soluble Pd(II).[1] A closely related compound is bis(acetonitrile)palladium dichloride.
The complex is prepared by dissolving PdCl2 in warm benzonitrile.[2] The PhCN ligands are labile, and the complex reverts to PdCl2 in noncoordinating solvents. According to X-ray crystallography, the two PhCN ligands are mutually trans.[3]

References
- ^ Jiro Tsuji; Hao Guo; Shengming Ma; Daniela Sustac Roman (2015). "Bis(benzonitrile)dichloropalladium(II)". e-EROS Encyclopedia of Reagents for Organic Synthesis. pp. 1–27. doi:10.1002/047084289X.rb101.pub3. ISBN 978-0-470-84289-8.
- ^ Gordon K. Anderson; Minren Lin (2007). "Bis(Benzonitrile)Dichloro Complexes of Palladium and Platinum". Inorganic Syntheses. Vol. 28. pp. 60–63. doi:10.1002/9780470132593.ch13. ISBN 978-0-470-13259-3.
{{cite book}}:|journal=ignored (help) - ^ Olmstead, M. M.; Wei, P.-P.; Ginwalla, A. S.; Balch, A. L. (2000). "Bis(Benzonitrile)Palladium(II) Dihalides: Structures and Cocrystallization of the Cubic Cluster Pd6Cl12 with (E)-Stilbene and with Bis(Benzonitrile)Palladium(II) Dichloride". Inorganic Chemistry. 39 (20): 4555–4559. doi:10.1021/ic0000597.
Categories:
- CS1 errors: periodical ignored
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical pages without ChemSpiderID
- Articles without EBI source
- Articles without KEGG source
- Articles without UNII source
- ECHA InfoCard ID from Wikidata
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Articles with short description
- Short description matches Wikidata
- Palladium compounds
- Homogeneous catalysis
- Coordination complexes
- Chloro complexes
- Benzonitriles