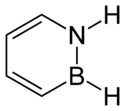
1,2-Dihydro-1,2-azaborine
Jump to navigation
Jump to search
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Dihydro-1,2-azaborine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H6BN | |||
| Molar mass | 78.908 g mol−1 | ||
| Appearance | clear, colorless liquid | ||
| Melting point | −46 to −45 °C. | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,2-Dihydro-1,2-azaborine is an aromatic chemical compound with properties intermediate between benzene and borazine. Its chemical formula is C4BNH6. It resembles a benzene ring, except that two adjacent carbons are replaced by nitrogen and boron, respectively.
Preparation
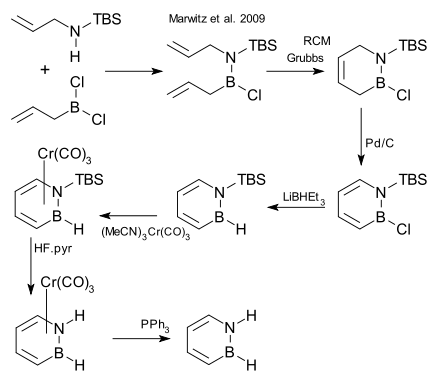
After decades of failed attempts, the compound was synthesized in 2008 and reported in January 2009.[1][2]
One of the synthetic steps is a ring-closing metathesis (RCM) reaction:[3]
References
- ^ Stu Borman. "Long-Sought Benzenelike Molecule Created: Aromaticity of organic-inorganic hybrid resembles benzene's." C&EN January 5, 2009 Volume 87, Number 01 p. 11
- ^ A. J. V. Marwitz; M. H. Matus; L. N. Zakharov; D. A. Dixon; S.-Y. Liu (January 2009). "A Hybrid Organic/Inorganic Benzene". Angew. Chem. Int. Ed. 48 (5): 973–977. doi:10.1002/anie.200805554. PMID 19105174.
- ^ TBS = tert-butyldimethylsilyl, step 2 RCM = ring-closing metathesis using Grubbs' catalyst, step 3 organic oxidation using palladium on carbon, step 4 reduction LiBHEt3, step 5 conversion to piano stool complex as protective group with chromium carbonyl derivative, step 6 cleavage N-TBS bond HF, step 7 deprotection with triphenylphosphine
Categories:
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Articles without UNII source
- Articles with changed CASNo identifier
- Articles with changed ChemSpider identifier
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Aromatic compounds
- Boron heterocycles
- Nitrogen heterocycles
- Six-membered rings
- Boron–nitrogen compounds