File:TACG A 178546 O F0001g.jpg
Jump to navigation
Jump to search
Size of this preview: 605 × 599 pixels. Other resolutions: 242 × 240 pixels | 485 × 480 pixels | 750 × 743 pixels.
Original file (750 × 743 pixels, file size: 259 KB, MIME type: image/jpeg)
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
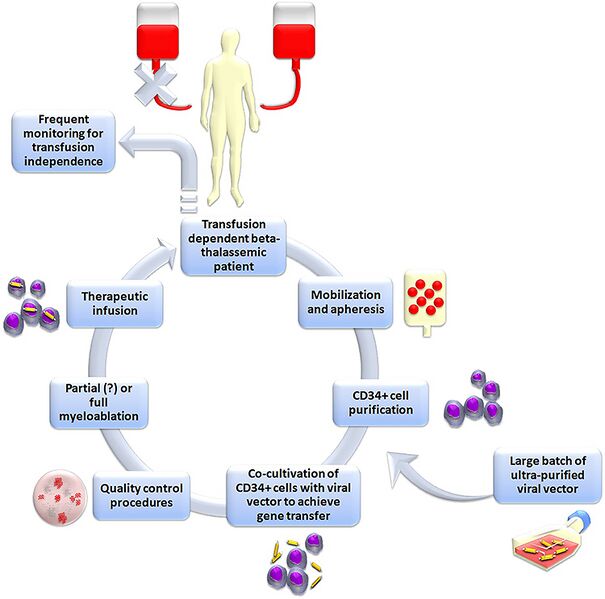
| current | 21:41, 26 December 2023 |  | 750 × 743 (259 KB) | Ozzie10aaaa | Uploaded a work by Karponi G, Zogas N from https://www.dovepress.com/gene-therapy-for-beta-thalassemia-updated-perspectives-peer-reviewed-fulltext-article-TACG with UploadWizard |
File usage
The following page uses this file: