File:PMC3119412 ART2011-354908.002.png
Jump to navigation
Jump to search
PMC3119412_ART2011-354908.002.png (512 × 378 pixels, file size: 160 KB, MIME type: image/png)
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
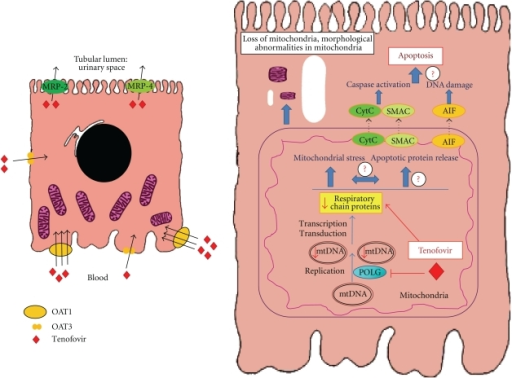
| current | 20:43, 10 May 2023 |  | 512 × 378 (160 KB) | Ozzie10aaaa | Uploaded a work by Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Niño MD, Izquierdo MC, Poveda J, Sainz-Prestel V, Ortiz-Martin N, Parra-Rodriguez A, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A from https://openi.nlm.nih.gov/detailedresult?img=PMC3119412_ART2011-354908.002&query=Tenofovir%20disoproxil&it=xg&req=4&npos=1 with UploadWizard |
File usage
There are no pages that use this file.