Dihydrotanshinone I
(Redirected from Dihydrotanshinone)
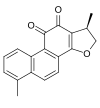 2D Structure
| |
 3D Conformer
| |
| Names | |
|---|---|
| IUPAC name
4,17β-Dimethyl-15-oxagona-1,3,5,7,9,13-hexaene-11,12-dione
| |
| Systematic IUPAC name
(1R)-1,6-Dimethyl-1,2-dihydrophenanthro[1,2-b]furan-10,11-dione | |
| Other names
15,16-dihydrotanshinone I, phenanthro[1,2-b]furan-10,11-dione, 1,2-dihydro-1,6-dimethyl-
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | DI |
| ChemSpider | |
| ECHA InfoCard | 100.222.905 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H14O3 | |
| Molar mass | 278.307 g·mol−1 |
| Appearance | Red powder |
| Density | 1.32 g/cm3 |
| Boiling point | 479.2 °C (894.6 °F; 752.3 K) |
| 12.9 mg/L (est.) | |
| Solubility in ethanol | 1 mg/mL, clear orange to red |
| log P | log Kow = 3.93 (est) |
| Vapor pressure | 3.41x10−9 mmHg |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H400 | |
| P264, P270, P273, P301+P312, P330, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dihydrotanshinone I (DI) is a naturally occurring compound extracted from Salvia miltiorrhiza Bunge, also known as Chinese sage, red sage root, and the Chinese herbal Dan Shen. It belongs to a class of lipophilic abietane diterpenoids[2] and has been reported to have cytotoxicity to a variety of tumor cells,[3] as well as antiviral effects in vitro.[4] Since they were first discovered, over 40 related compounds and over 50 hydrophilic compounds have been isolated from Dan Shen.[2]
References
- ^ "Dihydrotanshinone I". PubChem. PubChem. Retrieved 31 August 2015.
- ^ a b "HSDB: DIHYDROTANSHINONE I". NIH. Retrieved 1 September 2015.
- ^ Bian W, Chen F, Bai L, Zhang P, Qin W (January 2008). "Dihydrotanshinone I inhibits angiogenesis both in vitro and in vivo". Acta Biochimica et Biophysica Sinica. 40 (1): 1–6. doi:10.1111/j.1745-7270.2008.00370.x. PMID 18180848.
- ^ Lim CT, Tan KW, Wu M, Ulferts R, Armstrong LA, Ozono E, Drury LS, Milligan JC, Zeisner TU, Zeng J, Weissmann F, Canal B, Bineva-Todd G, Howell M, O'Reilly N, Beale R, Kulathu Y, Labib K, Diffley JF (July 2021). "Identifying SARS-CoV-2 antiviral compounds by screening for small molecule inhibitors of Nsp3 papain-like protease". The Biochemical Journal. 478 (13): 2517–2531. doi:10.1042/BCJ20210244. PMC 8286840. PMID 34198325.