Phendimetrazine
 | |
| Names | |
|---|---|
| Trade names | Bontril, Adipost, Anorex-SR, others |
| |
| Clinical data | |
| Drug class | Amphetamine[1] |
| Main uses | Obesity[1] |
| Side effects | Palpitations, fast heart rate, high blood pressure, agitation, trouble sleeping, headache, psychosis, diarrhea[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Peak plasma levels occur within 1 to 3 hours. Absorption is usually complete by 4 to 6 hours |
| Metabolism | Liver |
| Elimination half-life | 19-24 hours |
| Excretion | Urinary elimination |
| Chemical and physical data | |
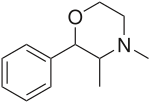
| Formula | C12H17NO |
| Molar mass | 191.274 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Phendimetrazine, sold under the brand name Bontril among others, is a medication used to treat obesity.[1] Use is only recommended for a few weeks.[1] It is used together with dieting and exercise.[1][2] It is taken by mouth.[1]
Common side effects include palpitations, fast heart rate, high blood pressure, agitation, trouble sleeping, headache, psychosis, and diarrhea.[1] Other side effects may include abuse, valvular heart disease, and pulmonary hypertension.[1] Use during pregnancy may harm the baby.[3] It is an amphetamine which works by decreasing appetite.[1]
Phendimetrazine was approved for medical use in the United States in 1975.[1] Europe voted to removal approval in 1999.[4] It is available as a generic medication.[2] In the United States 60 tablets of 35 mg costs about 10 USD as of 2021.[2] In the United States it is a Schedule III controlled substance.[5]
Medical uses
Dosage
For obesity in adults, it is taken at a dose of 35 mg immediate release two to three times per day.[1] A long acting formulation may be taken as 105 mg once per day.[1]
Pharmacology
It is a stimulant drug of the morpholine chemical class used as an appetite suppressant.[6]
Phendimetrazine functions as a prodrug to phenmetrazine; approximately 30 percent of an oral dose is converted into it. Phendimetrazine can essentially be thought of as an extended-release formulation of phenmetrazine with less potential for abuse. Phendimetrazine is an anorectic drug which acts as a norepinephrine-dopamine releasing agent (NDRA).[7]
As an amphetamine congener, its structure incorporates the backbone of methamphetamine, a potent CNS stimulant. While the addition of an N-methyl group to amphetamine significantly increases its potency and bioavailability, methylation of phenmetrazine renders the compound virtually inactive. However, phendimetrazine is a prodrug for phenmetrazine which acts as the active metabolite. Phendimetrazine possesses preferable pharmacokinetics over phenmetrazine as a therapeutic agent because its metabolization by demethylases produces a more steady and prolonged exposure of active drug within the body. This decreases abuse potential as the peak blood-concentration of active phenmetrazine that's produced from a single dose of phendimetrazine is lower than a single therapeutically equivalent dose of phenmetrazine.
Indicated as a short-term secondary treatment for exogenous obesity, phendimetrazine immediate-release 35mg tablets are typically consumed one hour before meals, not to exceed three doses daily. Phendimetrazine is also manufactured as a 105mg extended-release capsule for once daily dosing, typically consumed 30 to 60 minutes before a morning meal. Whereas the immediate-release formulation has a maximum daily dosage of 210mg (6 tablets), the extended-release capsules have a maximum daily dosage of 105mg (one capsule).
Society and culture
Names
Adipost, Anorex-SR, Appecon, Melfiat, Obezine, Phendiet, Plegine, Prelu-2, Statobex
Legality
According to the List of Psychotropic Substances under International Control Archived 2020-05-10 at the Wayback Machine published by the International Narcotics Control Board, phendimetrazine is a Schedule III controlled substance under the Convention on Psychotropic Substances.[8]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 "Phendimetrazine Monograph for Professionals". Drugs.com. Archived from the original on 27 January 2021. Retrieved 27 October 2021.
- ↑ 2.0 2.1 2.2 "Phendimetrazine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 6 February 2017. Retrieved 27 October 2021.
- ↑ "Phendimetrazine Use During Pregnancy". Drugs.com. Archived from the original on 5 December 2020. Retrieved 27 October 2021.
- ↑ "https://www.ema.europa.eu/en/documents/press-release/extraordinary-meeting-finalise-review-anorectic-agents_en.pdf" (PDF). Archived (PDF) from the original on 28 October 2021. Retrieved 27 October 2021.
{{cite web}}: External link in|title= - ↑ "PART 1308 - Section 1308.13 Schedule III". www.deadiversion.usdoj.gov. Archived from the original on 18 October 2021. Retrieved 27 October 2021.
- ↑ Landau D, Jackson J, Gonzalez G (2008). "A case of demand ischemia from phendimetrazine". Cases J. 1 (1): 105. doi:10.1186/1757-1626-1-105. PMC 2531092. PMID 18710555. Archived from the original on 2014-10-23. Retrieved 2020-09-26.
- ↑ Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–59. doi:10.2174/156802606778249766. PMID 17017961. Archived from the original on 2017-03-26. Retrieved 2020-05-05.
- ↑ "List of psychotropic substances under international control" (PDF). Archived from the original (PDF) on 2012-08-31. Retrieved 2020-09-26.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- CS1 errors: external links
- Articles with hatnote templates targeting a nonexistent page
- Missing redirects
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Webarchive template wayback links
- Drugs not assigned an ATC code
- Antiobesity drugs
- Anorectics
- Morpholines
- Euphoriants
- Norepinephrine-dopamine releasing agents
- RTT