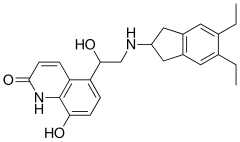
Indacaterol
 | |
 | |
| Names | |
|---|---|
| Trade names | Arcapta, Onbrez, others |
| Other names | Indacaterol maleate |
| |
| Clinical data | |
| Drug class | Long-acting beta-adrenoceptor agonist (LABA)[1] |
| Main uses | Chronic obstructive pulmonary disease (COPD)[1] |
| Side effects | Upper respiratory tract infection, chest pain, cough[2] |
| Pregnancy category |
|
| Routes of use | Inhalation |
| Typical dose | 75 to 300 mg OD[1][2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C24H28N2O3 |
| Molar mass | 392.499 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Indacaterol, sold under the brand name Onbrez among others, is a medication used to treat chronic obstructive pulmonary disease (COPD).[1] Is is used by dry powder inhaler once per day.[1]
Common side effects include upper respiratory tract infection, chest pain, and cough.[2] In those with asthma it may increase the risk of death.[1] It is a long-acting beta-adrenoceptor agonist (LABA).[1]
Indacaterol was approved for medical use in Europe in 2009 and the United States in 2011.[2][1] In the United Kingdom it costs the NHS about £32 a month as of 2021.[7] In the United States this amount costs about 260 USD.[8]
Medical uses
A Cochrane review found benefit in lung function in people with COPD at least as good as that seen with twice-daily long-acting beta2-agonists.[9]
Dosage
It is used at a dose of 75 to 300 mg once per day.[1][2]
History
It was approved by the European Medicines Agency (EMA) under the brand name Onbrez Breezhaler on November 30, 2009,[10] and by the United States Food and Drug Administration (FDA), under the brand name Arcapta Neohaler, on July 1, 2011.[11][12] In 2016, Novartis licensed its U.S. commercial rights for Arcapta Neohaler to Sunovion Pharmaceuticals.[13]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Indacaterol Monograph for Professionals". Drugs.com. Archived from the original on 22 January 2021. Retrieved 26 November 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Onbrez Breezhaler". Archived from the original on 28 January 2021. Retrieved 26 November 2021.
- ↑ "Onbrez Breezhaler EPAR". European Medicines Agency (EMA). Archived from the original on 28 January 2021. Retrieved 20 January 2021.
- ↑ "Oslif Breezhaler EPAR". European Medicines Agency (EMA). Archived from the original on 28 January 2021. Retrieved 20 January 2021.
- ↑ "Hirobriz Breezhaler EPAR". European Medicines Agency (EMA). Archived from the original on 28 January 2021. Retrieved 20 January 2021.
- ↑ "Arcapta Neohaler (indacaterol) inhalation powder Initial U.S. Approval: 2011". DailyMed. 1 April 2020. Archived from the original on 15 June 2021. Retrieved 14 June 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 267. ISBN 978-0857114105.
- ↑ "Indacaterol Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 26 November 2021.
- ↑ Geake, James B (2015). "Indacaterol, a once-daily beta2-agonist, versus twice-daily beta2-agonists or placebo for chronic obstructive pulmonary disease". Reviews. 1: CD010139. doi:10.1002/14651858.CD010139.pub2. PMC 6464646. PMID 25575340.
- ↑ European Public Assessment Report for Onbrez Breezhaler EPARs for authorised medicinal products for human use - Onbrez Breezhaler at the Wayback Machine (archived 2010-01-16)
- ↑ "FDA approves Arcapta Neohaler to treat chronic obstructive pulmonary disease" (Press release). U.S. Food and Drug Administration. 2011-07-01. Archived from the original on 2011-07-03. Retrieved 2011-07-02.
- ↑ "Drug Approval Package: Arcapta Neohaler (indacaterol maleate) NDA #022383". U.S. Food and Drug Administration. 13 August 2013. Archived from the original on 7 April 2021. Retrieved 14 June 2021.
- Lay summary in: (PDF) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022383Orig1s000SumR.pdf.
{{cite web}}: Missing or empty|title=(help)
- Lay summary in: (PDF) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022383Orig1s000SumR.pdf.
- ↑ Faulkner, Sarah (22 December 2016). "Sunovion, Novartis ink licensing deal for inhaled COPD drugs". Drug Delivery Business. Archived from the original on 31 October 2021. Retrieved 15 June 2021.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1 errors: missing title
- CS1 errors: bare URL
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- 2-Aminoindanes
- Phenylethanolamines
- Long-acting beta2-adrenergic agonists
- Novartis brands
- Quinolinols
- 2-Quinolones
- RTT