Fostemsavir
 | |
 | |
| Names | |
|---|---|
| Trade names | Rukobia |
| Other names | Fostemsavir tromethamine, BMS-663068, GSK3684934 |
| |
| Clinical data | |
| Drug class | HIV fusion inhibitor[1] |
| Main uses | HIV/AIDS[2] |
| Side effects | Nausea, diarrhea, rash, abdominal pain[3][2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 600 mg BID[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620046 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
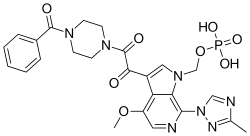
| Formula | C25H26N7O8P |
| Molar mass | 583.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fostemsavir, sold under the brand name Rukobia, is a medication used to treat HIV/AIDS.[2] It is used together with other medications, when usually treatments are not effective.[3] It is taken by mouth.[3]
Common side effects include nausea, diarrhea, rash, and abdominal pain.[3][2] Other side effects may include liver problems, QT prolongation, and immune reconstitution syndrome.[3] It should not be taken with strong CYP3A inducers.[2] It works by binding to the HIV virus and preventing it from entering T cells.[2]
Fostemsavir was approved for medical use in the United States 2020, and Europe in 2021.[1][2] In the United Kingdom a month of treatment costs the NHS £2,900 as of 2021.[5] In the United States this amount costs about 8,000 USD.[6]
Medical uses
Fostemsavir in combination with other antiretroviral(s), is indicated for the treatment of HIV-1 infection in heavily treatment-experienced adults with multidrug-resistant HIV-1 infection failing their current antiretroviral regimen due to resistance, intolerance, or safety considerations.[7]
Dosage
It is taken at a dose of 600 mg twice per day.[3]
Side effects
Fostemsavir may cause a serious condition called immune reconstitution syndrome, similar to other approved drugs for treatment of HIV-1 infection.[8] This condition can happen at the beginning of HIV-1 treatment when the immune system may get stronger and begin to fight infections that have been hidden in the body for a long time.[8] Other serious side effects include heart rhythm problems due to prolongation of heart electrical activity (QT prolongation) and an increase of liver enzymes in patients with hepatitis B or C virus co-infection.[8]
Mechanism of action
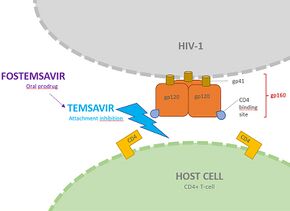
In terms of the mode of action we find that Fostemsavir binds to gp120 subunit within HIV-1 gp160 envelope glycoprotein, this in turn inhibits the activity of gp120(this subunit makes possible the attachment of HIV-1 to host CD4 receptor)[9]
History
It was under development by ViiV Healthcare / GlaxoSmithKline for use in the treatment of HIV infection. By blocking the gp120 receptor of the virus, it prevents initial viral attachment to the host CD4+ T cell and entry into the host immune cell; its method of action is a first for HIV drugs.[10] Because it targets a different step of the viral lifecycle, it offers promise for individuals with virus that has become highly resistant to other HIV drugs.[11] Since gp120 is a highly conserved area of the virus, the drug is unlikely to promote resistance to itself.[12] Investigators found that enfuvirtide-resistant and ibalizumab-resistant HIV envelopes remained susceptible to Fostemsavir. Conversely, Fostemsavir-resistant HIV remained susceptible to all the entry inhibitors. Furthermore, HIV isolates that do not require the CD4 receptor for cell entry were also susceptible to Fostemsavir, and the virus did not escape the attachment inhibitor by becoming CD4-independent. Prior in vitro studies showed that Fostemsavir inhibits both CCR5-tropic and CXCR4-tropic HIV.[10]

Fostemsavir was approved for medical use in the United States in July 2020.[13][8][7]
The safety and efficacy of fostemsavir, taken twice daily by mouth, were evaluated in a clinical trial of 371 heavily treatment-experienced adult participants who continued to have high levels of virus (HIV-RNA) in their blood despite being on antiretroviral drugs.[13] Two hundred seventy-two participants were treated in the main trial arm, and an additional 99 participants received fostemsavir in a different arm of the trial.[13][8] Most participants had been treated for HIV for more than 15 years (71 percent), had been exposed to five or more different HIV treatment regimens before entering the trial (85 percent) and/or had a history of AIDS (86 percent).[13] Participants in the main cohort of the trial received either fostemsavir or a placebo twice daily for eight days, in addition to their failing antiretroviral regimen.[13][8] On the eighth day, participants treated with fostemsavir had a significantly greater decrease in levels of HIV-RNA in their blood compared to those taking the placebo.[13] After the eighth day, all participants received fostemsavir with other antiretroviral drugs.[13][8] After 24 weeks of fostemsavir plus other antiretroviral drugs, 53 percent of participants achieved HIV RNA suppression, where levels of HIV were low enough to be considered undetectable.[13] After 96 weeks, 60 percent of participants continued to have HIV RNA suppression.[13] The clinical trial (NCT02362503) was conducted at 108 sites in 23 countries in North America, South America, Europe, Australia, Taiwan and South Africa.[8]
The U.S. Food and Drug Administration (FDA) granted the application for fostemsavir fast track, priority review, and breakthrough therapy designations.[13] The FDA granted the approval of Rukobia to ViiV Healthcare.[13]
On 10 December 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Rukobia, intended for the treatment of multi-drug resistant HIV-1 infection.[14] The applicant for this medicinal product is ViiV Healthcare B.V. Fostemsavir was approved for medical use in the European Union in February 2021.[2]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[15]
References
- ↑ 1.0 1.1 "Fostemsavir Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 12 December 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Rukobia EPAR". European Medicines Agency (EMA). 9 December 2020. Archived from the original on 12 February 2021. Retrieved 12 February 2021.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Rukobia- fostemsavir tromethamine tablet, film coated, extended release". DailyMed. 2 July 2020. Archived from the original on 15 July 2020. Retrieved 14 July 2020.
- ↑ 4.0 4.1 "Rukobia". Therapeutic Goods Administration (TGA). 23 July 2021. Archived from the original on 5 September 2021. Retrieved 5 September 2021.
- ↑ "Rukobia · HIV infection". Retrieved 13 December 2021.
{{cite web}}: CS1 maint: url-status (link) - ↑ "Rukobia Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 13 December 2021.
- ↑ 7.0 7.1 "ViiV Healthcare Announces US FDA Approval for Rukobia (fostemsavir), a First-in-Class Treatment for HIV in Adults With Few Treatment Options Available" (Press release). ViiV Healthcare. 2 July 2020. Archived from the original on 3 July 2020. Retrieved 2 July 2020 – via Business Wire.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 "Drug Trials Snapshots: Rukobia". U.S. Food and Drug Administration (FDA). 2 July 2020. Archived from the original on 31 July 2020. Retrieved 14 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 9.0 9.1 Muccini, Camilla; Canetti, Diana; Castagna, Antonella; Spagnuolo, Vincenzo (25 January 2022). "Efficacy and Safety Profile of Fostemsavir for the Treatment of People with Human Immunodeficiency Virus-1 (HIV-1): Current Evidence and Place in Therapy". Drug Design, Development and Therapy. 16: 297–304. doi:10.2147/DDDT.S273660. Archived from the original on 8 April 2024. Retrieved 1 May 2024.
- ↑ 10.0 10.1 "HIV Attachment Inhibitor BMS-663068 Looks Good in Early Studies". Archived from the original on 2 June 2021. Retrieved 5 September 2021.
- ↑ "HIV attachment inhibitor BMS-663068 shows good safety and efficacy in phase 2b study". Archived from the original on 1 November 2018. Retrieved 5 September 2021.
- ↑ "Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors". Archived from the original on 1 November 2018. Retrieved 5 September 2021.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 "FDA Approves New HIV Treatment for Patients With Limited Treatment Options". U.S. Food and Drug Administration (Press release). 2 July 2020. Archived from the original on 3 July 2020. Retrieved 2 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Rukobia: Pending EC decision". European Medicines Agency (EMA). 11 December 2020. Archived from the original on 12 December 2020. Retrieved 11 December 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Archived from the original on 18 January 2021. Retrieved 17 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Kozal M, Aberg J, Pialoux G, Cahn P, Thompson M, Molina JM, et al. (March 2020). "Fostemsavir in Adults with Multidrug-Resistant HIV-1 Infection". N. Engl. J. Med. 382 (13): 1232–1243. doi:10.1056/NEJMoa1902493. PMID 32212519.
- Pages using duplicate arguments in template calls
- CS1 maint: url-status
- Wikipedia articles incorporating the PD-notice template
- Use dmy dates from July 2020
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Breakthrough therapy
- Entry inhibitors
- Organophosphates
- Piperazines
- Prodrugs
- Pyrrolopyridines
- Triazoles
- RTT