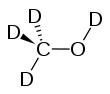
Deuterated methanol
Jump to navigation
Jump to search

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(2H3)Methan(2H)ol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1733278 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.011.253 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UN number | 1230 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CD4O | |||
| Molar mass | 36.0665 g mol−1 | ||
| Density | 0.888 g cm−3 | ||
| Melting point | −98 °C (−144 °F; 175 K) | ||
| Boiling point | 65 °C (149 °F; 338 K) | ||
| Thermochemistry | |||
Heat capacity (C)
|
87.9 J K−1 mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Warning | |||
| H225, H301, H311, H331, H370 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P310, P302+P352, P303+P361+P353, P304+P340, P307+P311, P311, P312, P321, P322, P330, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| Flash point | 11 °C (52 °F; 284 K) | ||
| Related compounds | |||
Related compounds
|
Methanol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Deuterated methanol (CD3OD), is a form (called an isotopologue) of methanol (CH3OH) in which the hydrogen atoms ("H") are replaced with deuterium (heavy hydrogen) isotope ("D").[1] Deuterated methanol is a common solvent used in NMR spectroscopy.
Deuterated methanol was first detected in interstellar space was Orion-KL in 1988 by scientists at the Max Planck Institute for Radio Astronomy.[2]
References
- ^ Bizzocchi, L.; Caselli, P.; Spezzano, S.; Leonardo, E. (2014-09-01). "Deuterated methanol in the pre-stellar core L1544". Astronomy & Astrophysics. 569: A27. arXiv:1408.2491. Bibcode:2014A&A...569A..27B. doi:10.1051/0004-6361/201423858. ISSN 0004-6361.
- ^ Mauersberger, R.; Henkel, C.; Jacq, T.; Walmsley, C. M. (1988-04-01). "Deuterated methanol in Orion". Astronomy and Astrophysics. 194: L1–L4. Bibcode:1988A&A...194L...1M. ISSN 0004-6361.
Categories:
- Articles with short description
- Short description matches Wikidata
- Articles without InChI source
- Articles without KEGG source
- Articles without UNII source
- ECHA InfoCard ID from Wikidata
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Deuterated solvents
- Methanol
- All stub articles
- Nuclear magnetic resonance stubs
- Organic compound stubs