Zavegepant
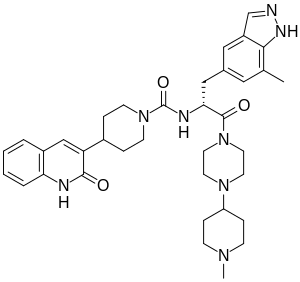 | |
| Names | |
|---|---|
| Trade names | Zavzpret |
| Other names | BHV-3500 |
| |
| Clinical data | |
| Drug class | Calcitonin gene-related peptide receptor antagonist[1] |
| Main uses | Migraine headaches[1] |
| Side effects | Taste changes, nausea, sore nose, vomiting[1] |
| Routes of use | Nasal |
| Typical dose | 10 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C36H46N8O3 |
| Molar mass | 638.817 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zavegepant, sold under the brand name Zavzpret, is a medication used to treat migraine headaches.[1] It is used for the headache itself, rather than prevention.[1] It is sprayed into the nose.[1]
Common side effects include taste changes, nausea, sore nose, and vomiting.[1] Other side effects include allergic reactions.[1] Use is not recommended in people with significant liver or kidney problems.[1] Safety in pregnancy is unclear.[1] It is a calcitonin gene-related peptide receptor antagonist.[1]
Zavegepant was approved for medical use in the United States in 2023.[1] It is expected to become commercially available in July of 2023 and be priced similar to other medications in its class.[2]
Medical uses
Zavegepant is indicated for the acute treatment of migraine with or without aura in adults.[1]
Dosage
It is given at a dose of 10 mg, with at most one dose per day.[1]
Society and culture
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 "ZAVZPRET™ (zavegepant) nasal spray" (PDF). Archived (PDF) from the original on 2023-03-11. Retrieved 2023-03-13.
- ↑ MSc, Nadia Stec (14 March 2023). "Zavzpret (Zavegepant) Migraine Nasal Spray from Pfizer Gets FDA Nod". Xtalks. Archived from the original on 15 March 2023. Retrieved 25 May 2023.
External links
| Identifiers: |
|
|---|
- Noor N, Angelette A, Lawson A, Patel A, Urits I, Viswanath O, et al. (2022). "A Comprehensive Review of Zavegepant as Abortive Treatment for Migraine". Health Psychology Research. 10 (3): 35506. doi:10.52965/001c.35506. PMC 9239361. PMID 35774914.
- Scuteri D, Tarsitano A, Tonin P, Bagetta G, Corasaniti MT (November 2022). "Focus on zavegepant: the first intranasal third-generation gepant". Pain Management. 12 (8): 879–885. doi:10.2217/pmt-2022-0054. PMID 36189708. S2CID 252681912.
- Clinical trial number NCT04571060 for "Randomized Trial in Adult Subjects With Acute Migraines" at ClinicalTrials.gov
- Clinical trial number NCT03872453 for "Acute Treatment Trial in Adult Subjects With Migraines" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Multiple chemicals in Infobox drug
- Chemicals using indexlabels
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs not assigned an ATC code
- Antimigraine drugs
- Calcitonin gene-related peptide receptor antagonists
- Pfizer brands
- Piperazines
- Piperidines
- Indazoles
- Quinolines
- Ureas
- RTT