Visceral leishmaniasis
| Visceral leishmaniasis | |
|---|---|
| Other names: Kala-azar;[1] black fever;[2] dum-dum fever[3] | |
 | |
| Leishmania donovani | |
| Pronunciation |
|
| Symptoms | Fever, weight loss, enlarged liver and spleen[1] |
| Complications | Low red blood cells, post-kala-azar dermal leishmaniasis[1] |
| Causes | L. donovani, L. infantum[4] |
| Diagnostic method | Based on symptoms supported by blood tests[1] |
| Prevention | Insect repellant, insect nets[1] |
| Treatment | Liposomal amphotericin B; sodium stibogluconate and paromomycin[4] |
| Frequency | 50,000 to 90,000 per year[1] |
| Deaths | 6,000 (2019)[5] |
Visceral leishmaniasis (VL), also known as kala-azar, is the most severe form of leishmaniasis.[1] Symptoms may include fever, weight loss, low red blood cells, and an enlarged liver and spleen.[1] Complications may include post-kala-azar dermal leishmaniasis.[1]
It is caused by parasites of the Leishmania type, specifically L. donovani in Asia and Africa and L. infantum in South America and the Middle East.[2][4] It is spread by certain types of sandflies.[1] Risk factors for severe disease include HIV.[6] Diagnosis is based on symptoms and supported by blood tests.[1]
Efforts to prevent the disease include eliminating or avoiding sandflies.[1] Treatment is often with liposomal amphotericin B; however, in African sodium stibogluconate and paromomycin work better, though have greater side effects.[4] Miltefosine may also be used.[4] Without treatment, death typically occurs.[1]
Visceral leishmaniasis most commonly occurs in India, east Africa, and Brazil.[1] Often cases occur as outbreaks, newly affecting about 50,000 to 90,000 people a year.[1] In 2019 it resulted in about 6,000 deaths.[5] Effective treatment become available in 1922 when Upendranath Brahmachari made urea stibamine.[7]
Signs and symptoms

When people develop visceral leishmaniasis, the most typical symptoms are fever and the enlargement of the spleen, with enlargement of the liver sometimes being seen as well.[8]
The darkening of the skin that gave the disease its common name in India does not appear in most strains of the disease, and the other symptoms are very easy to mistake for those of malaria. Without proper treatment the mortality rate for kala-azar is very high; progress of the disease is extremely variable, taking anywhere from one to twenty weeks, but a typical duration for the Sudanese strain of the disease is narrower, between twelve and sixteen weeks.[9][10][11]
L. donovani itself is not usually the direct cause of death in kala-azar sufferers, however. Tuberculosis is omnipresent in the immuno-depressed regions where leishmaniasis thrives, and, as with AIDS, it is these opportunistic infections that are more likely to kill, flaring up in a host whose immune system has been weakened by the L. donovani infection. [12][13]
Complications
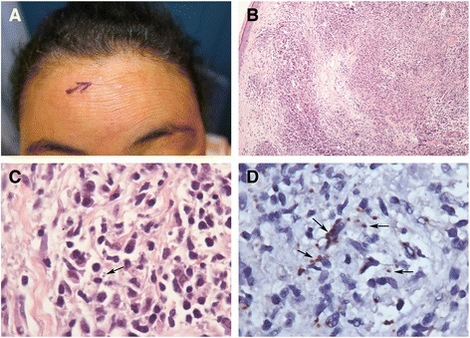
Even with recovery, kala-azar does not always leave its hosts unmarked. Some time after successful treatment—generally a few months with African kala-azar, or as much as several years with the Indian strain—a secondary form of the disease may set in. This condition manifests first as small, measle-like skin lesions on the face, which gradually increase in size and spread over the body. Eventually the lesions may coalesce to form disfiguring, swollen structures resembling leprosy, and occasionally causing blindness if they spread to the eyes. (This disease is not the same as cutaneous leishmaniasis, a milder disease caused by another protozoan of the Leishmania genus which also causes skin lesions.)[15]
Post-kala-azar dermal leishmaniasis (PKDL) is a recurrence of leishmaniasis that appears on the skin of affected individuals months and up to 20 years after being partially treated, untreated or even in those considered adequately treated.[16][17] In Sudan, they can be demonstrated in up to 60% of treated cases. They manifest as hypopigmented skin lesions (such as macules, papules, nodules), or facial redness. Though any organism causing kala-azar can lead to PKDL, it is commonly associated with Leishmania donovani which gives different disease patterns in India and Sudan. In the Indian variant, nodules enlarge with time and form plaques but rarely ulcerate, but nodules from the African variety often ulcerate as they progress. Nerve involvement is common in African variety but rare in Indian subcontinent.[18] Histology demonstrates a mixture of chronic inflammatory cells; there can be macrophage or epitheloid granuloma.[19]
Newer polymerase chain reaction (PCR) based tools have higher sensitivity and specificity. Emergence of PKDL has been reported in HIV affected individuals[20] and may become a problem in the future.
Sodium stibogluconate alone or in combination with rifampicin is used for the treatment of PKDL for a long course of several months, compliance may be an issue for such a long course.[21]
Cause
Two species of Leishmania are known to give rise to the visceral form of the disease. The species commonly found in East Africa and the Indian subcontinent is L. donovani and the species found in Europe, North Africa, and Latin America is L. infantum, also known as L. chagasi.[22]
The insect vectors are species of sandfly of the genus Phlebotomus in the Old World, and of Lutzomyia in the New World. Sandflies are tiny flies, measuring 3–6 mm long by 1.5–3 mm in diameter, and are found in tropical or temperate regions throughout the world. The sandfly species Lutzomyia longipalpis is the primary vector of this disease. The larvae grow in warm, moist organic matter making them hard to eradicate.[23][24][25]
Visceral Leishmaniasis/kala-azar samples from India revealed the presence of not only the primary causative protozoan parasite, i.e. Leishmania donovani (LD) but also co-infection with another protozoan member called Leptomonas seymouri (LS). The latter parasite (LS) further contained a RNA virus known as Leptomonas seymouri narna-like virus 1 (Lepsey NLV1). So, it appears that a great majority of kala-azar victims in the Indian subcontinent are exposed to a RNA virus in LS, the co-infecting parasite with LD i.e. the "LD-LS-Lepsey NLV1 triple pathogen" phenomenon.[26]
-
Lutzomyia longipalpis sandfly
-
Leishmania donovani
Life cycle

The life cycle of Leishmania is completed in two hosts, humans and sandflies. The adult female sandfly is a bloodsucker, usually feeding at night on sleeping prey. When the fly bites an individual infected with Leishmania, the pathogen is ingested along with the prey's blood. The protozoan is in the smaller of its two forms, called an amastigote, which is round, non-motile, and only 3–7 micrometers in diameter. Inside the stomach of the sandfly, the amastigotes quickly transform into elongated and motile forms called the promastigotes. Promastigote is spindle-shaped, triple the size of the amastigote, and has a single flagellum that allows mobility. The promastigotes live extracellularly in the alimentary canal, reproducing asexually, then migrate to the proximal end of the gut where they become poised for a regurgitational transmission. As the fly bites, the promastigotes are released from the proboscis and introduced locally at the bite site.[27][28][29]
Once inside the human host, promastigotes invade macrophages. Inside the cells they transform back into the smaller amastigote form. The amastigotes replicate in the most hostile part of the macrophage cell, inside the phagolysosome, whose normal defensive response they are able to prevent. After repeated multiplication, they break down their host cell by sheer pressure of mass, but there is some recent speculation that they are able to leave the cell by triggering the exocytosis response of the macrophage.[30] The daughter cells protozoans then migrate to fresh cells or through the bloodstream to find new hosts. In this way the infection is progressive, spreading to the host's mononuclear phagocyte system, particularly the spleen and liver. The free amastigotes in peripheral tissues are then ingested by sandfly to enter another cycle.[31][32][33][34]
Mechanism
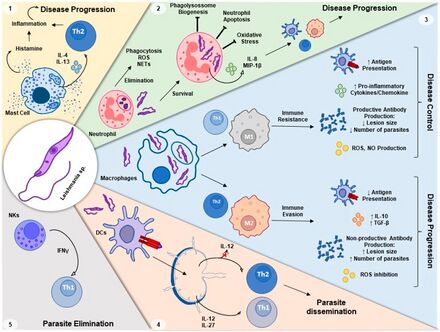
The cell-mediated immunity that kills Leishmania also produces inflammation. If the inflammation is excessive, it can cause tissue damage. The role of regulatory T and regulatory B cells is to suppress CMI enough to prevent tissue damage. However, an excessive regulatory response can prevent clearance of Leishmania and could explain the anergy of VL, poor response to drug treatment, development of PKDL, and relapses. A role for regulatory cells in VL has long been suspected. A variety of regulatory T and B cells have been implicated in VL, including Type 1 T helper cells that secrete IL-10 in addition to IFN-γ, natural T reg, Tr1, CD8+ T reg, and B reg. All of these lymphocytes act, at least in part, by secreting IL-10 and other suppressive cytokines.[36][37][38][39]
CD4+ T regs are present at increased frequency in the bone marrow of VL patients, are one source of IL-10, and proliferate in response to Leishmania antigen.[40] Levels of FoxP3 mRNA were also up-regulated in lesional tissue from PKDL patients.[41] However, T regs are not elevated in spleen cells from VL patients nor does depletion of T regs increase Leishmania antigen specific IFN-γ secretion[42] The highest levels of IL-10 mRNA in spleen cells is in CD8+ and other non-FoxP3+ T cells.[43] White blood cell CD8+ T cells from VL patients have elevated IL-10 levels.[44] There is a 9.6 fold increase in IL-10 expressing CD8+ T cells among PBMC lymphocytes from PKDL patients.[41] In the one study of T cell clones from VL patients, the clones isolated from VL PBMC were 100% CD8+.[45] When mixed with self PBMC one or three years after successful treatment the CD8+ T cells decreased Leishmania antigen specific proliferation and IFN-γ secretion and increased IL-10 secretion. Depletion of CD8+ T cells from VL PBMC stopped endogenous IL-10 secretion but increased Leishmania antigen specific IL-10 secretion, suggesting that CD8+ regulatory T cells are responsible for endogenous IL-10 secretion.[46]
Regulatory B cells are known to favor development of regulatory T cells and suppress development of Type 1 T helper cells by producing IL-10 and other down-regulatory cytokines.[39] IL-10 levels are elevated in B cells from VL PBMC.[44] A study of dogs with naturally acquired VL showed that the percentage of regulatory B cells increased three-fold during VL,[47] depletion of B cells increased CD4+ T cell proliferation and IFN-γ secretion but decreased IL-10 secretion.
Diagnosis
The gold standard for diagnosis is visualization of the amastigotes in splenic aspirate or bone marrow aspirate.This is a technically challenging procedure that is frequently unavailable in areas of the world where visceral leishmaniasis is endemic.[48][49]
Serological testing is much more frequently used in areas where leishmaniasis is endemic. A 2014 Cochrane review evaluated different rapid diagnostic tests. One of them (the rK39 immunochromatographic test) gave correct, positive results in 92% of the people with visceral leishmaniasis and it gave correct, negative results in 92% of the people who did not have the disease. A second rapid test (called latex agglutination test) gave correct, positive results in 64% of the people with the disease and it gave correct, negative results in 93% of the people without the disease. Other types of tests have not been studied thoroughly enough to ascertain their efficacy.[50]
The K39 dipstick test is easy to perform, and village health workers can be easily trained to use it. The kit may be stored at ambient temperature and no additional equipment needs to be carried to remote areas. The DAT anti-leishmania antigen test, standard within MSF, is much more cumbersome to use and appears not to have any advantages over the K39 test.[51]
There are a number of problems with serological testing: in highly endemic areas, not everyone who becomes infected will actually develop clinical disease or require treatment. Indeed, up to 32% of the healthy population may test positive, but not require treatment.[52][53] Conversely, because serological tests look for an immune response and not for the organism itself, the test does not become negative after the patient is cured, it cannot be used as a check for cure, or to check for re-infection or relapse.[54] Likewise, patients with abnormal immune systems (e.g., HIV infection) will have false-negative tests.[55]Other tests being developed include detects erythrosalicylic acid.[54]
Prevention


As of 2018, there are no vaccines or preventive drugs for visceral leishmaniasis, but vaccines are in development.[56][57]
The most effective method to prevent infection is to protect from sand fly bites via the following:
1. Avoid outdoor activities, especially from dusk to dawn, when sand flies generally are the most active.
2. When outdoors (or in unprotected quarters), minimize the amount of exposed (uncovered) skin to the extent that is tolerable in the climate. Wear long-sleeved shirts, long pants, and socks; and tuck your shirt into your pants.
3. Apply insect repellent to exposed skin and under the ends of sleeves and pant legs. Follow the instructions on the label of the repellent. The most effective repellents generally are those that contain the chemical DEET (N,N-diethylmetatoluamide).
1. Stay in well-screened or air-conditioned areas.
2. Spray living/sleeping areas with an insecticide to kill insects.
3. If you are not sleeping in a well-screened or air-conditioned area, use a bed net and tuck it under your mattress, if possible, use a bed net that has been soaked in or sprayed with a pyrethroid-containing insecticide.
Treatments

As with many diseases in developing nations, (including trypanosomiasis and malaria) effective and affordable chemotherapy is sorely lacking and parasites or insect vectors are becoming increasingly resistant to existing anti-parasite drugs. Possibly due to the lack of financial return, new drugs are slow to emerge and much of the basic research into potential drug targets takes place in universities, funded by charitable organizations. Product Development Partnership, Drugs for Neglected Diseases initiative works on the development of new treatments (combination treatments and new chemical entities) for visceral leishmaniasis.[60]
The traditional treatment is with pentavalent antimonials such as sodium stibogluconate and meglumine antimoniate. Resistance is now common in India, and rates of resistance have been shown to be as high as 60% in parts of Bihar, India.[61][62]
The treatment of choice for visceral leishmaniasis acquired in India is now amphotericin B[63] in its various liposomal preparations.[64][65] In East Africa, the WHO recommended treatment is SSG&PM (sodium stibogluconate and paromomycin) developed by Drugs for Neglected Diseases initiative (DNDi) in 2010.[66]
Miltefosine is the first oral treatment for this disease. The cure rate of miltefosine in Phase III clinical trials is 95%; Studies in Ethiopia show that is also effective in Africa. In HIV immunosuppressed people which are coinfected with leishmaniasis it has shown that even in resistant cases 2/3 of the people responded to this new treatment. Miltefosine has received approval by the Indian regulatory authorities in 2002, in Germany in 2004 and in U.S.A. in 2014.[67]
The drug is generally better tolerated than other drugs. Main side effects are gastrointestinal disturbance in the first or second day of treatment which does not affect the efficacy. Because it is available as an oral formulation, the expense and inconvenience of hospitalization is avoided, and outpatient distribution of the drug becomes an option, making miltefosine a drug of choice. However, there are some important disadvantages. First, there is evidence of reduced efficacy after years of use and second, it is teratogenic and cannot be used in women of child-bearing age without anticonception.[68][69][70]
Incomplete treatment has been cited as a major reason of death from visceral leishmaniasis.[71] The nonprofit Institute for OneWorld Health has adopted the broad spectrum antibiotic paromomycin for use in treating VL; its antileishmanial properties were first identified in the 1980s. A treatment with paromomycin costs about US$15. The drug had originally been identified in the 1960s.[72] The Indian government approved paromomycin for sale and use in August 2006.[73]
Prognosis
Protective immunity
Immunity to Leishmania is determined by the interplay of white blood cells, cytokines, immune complexes, and genetic and environmental factors.[74] Protective immunity develops either after successful treatment of VL (cured) or after asymptomatic infections that resolve without development of VL (asymptomatic).[75][76] Both types of immunity are characterized by cell-mediated immunity (CMI), including skin test positivity, proliferation, and interleukin 2 (IL-2), interferon gamma (IFN-γ), and interleukin 12 (IL-12) secretion by peripheral blood mononuclear cells (PBMC) in response to Leishmania antigens.[77][78][79][80][81] T cells isolated from both cured and asymptomatic PBMC activate autologous macrophages to kill intracellular amastigotes.[82] IFN-γ activates macrophages to kill intracellular parasites so its role in VL has been studied extensively and IFN-γ production is often used as a marker of protective immunity. Cured PBMC generally secrete less IFN-γ and more interleukin 10 (IL-10) in response to Leishmania antigens than asymptomatic PBMC.[45] IL-12 is important in the development and maintenance of Type 1 T helper cell responses and protective immunity so its role in VL has also been studied. Addition of IL-12 to some VL PBMC increases proliferation and IFN-γ secretion in response to Leishmania antigens and anti-IL-12 inhibits proliferation and IFN-γ secretion by some cured PBMC.[81][83][84][85]
Leishmania antigen stimulation of PBMC from cured patients show a mixed T helper cell and regulatory T cell response.[86] Both CD4+ and CD8+ T cells contributed to IFN-γ production.[43][87] Studies of Leishmania antigen specific T cell clones from cured patient PBMC confirm that cured patients have a mixed T cell response that involves both CD4+ helper T cells and CD4+ and CD8+ regulatory T cells.[46][88][89] Two studies of asymptomatic T cell clones show that most have Type 1 profiles and secrete more IFN-γ than T cell clones from cured patients. Neither study revealed the presence of Type 2 or regulatory T cells.[45][89] Some clones secreted soluble factors that caused the death of CD8+ regulatory T cells but not CD4+ T cells from VL patients, which might explain the strong protective immunity of asymptomatic patients.[84]
Non-protective immunity
VL patients are unable to clear their infections because they lack CMI. This anergy may be limited to Leishmania antigens or extend to mitogens and other antigens as the disease progresses.[90] In addition to skin test negativity, VL patient PBMC do not proliferate or secrete IL-2 or IFN-γ in response to Leishmania antigens.[77][78][91] Memory T cells may be depleted in VL patient PBMC.[92][93] Since IL-10 is known to suppress innate and acquired immunity and prevent IFN-γ from activating macrophages, its role in VL has been studied extensively and elevated IL-10 production is often used as a marker of non-protective immunity in VL. Elevated levels of IL-10 in the plasma, infected tissues, and PBMC of VL patients accompany the anergy of VL.[94][95][96][97] PKDL patients also have elevated IL-10 levels.[41] VL patients with the highest IL-10 levels are more likely to be unresponsive to treatment and progress to PKDL.[40][98] PBMC secretion of IL-10 without the addition of Leishmania antigen (endogenous) is inversely correlated with antigen specific IFN-γ secretion but Leishmania antigen specific IL-10 and IFN-γ secretion are not correlated, suggesting that endogenous secretion is more important in pathology.[46] Addition of anti-IL-10 increases proliferation and IFN-γ secretion by PBMC from some patients.[80][84] Both CD4+ and CD8+ T cells have been shown to contribute to IL-10 secretion by VL PBMC.[44][87] The high level of immune complexes characteristic of VL have also been shown to increase IL-10 levels.[99]
Epidemiology
More than 90% of the occurred in seven countries in 2015: Brazil, Ethiopia, India, Kenya, Somalia, South Sudan and Sudan.[2] Cases; however, occurred in at least 60 countries.[2] The number of people newly infected each year has decreased from about 30,000 in 2012 to 70,000 in 2017.[2]
In India, a high percentage of VL cases are reported from the state of Bihar.North Bihar, India is the endemic zone of this disease. The disease is endemic in more than 60 countries. In Iran this includes Ardabil, Fars, and North Khorasan.[71][100]
However, while the disease's geographical range is broad, it is not continuous. The disease clusters around areas of drought, famine, and high population density. In Africa, this has meant a knot of infection centers mostly in South Sudan, Sudan, Ethiopia, Kenya, and Somalia.[101]
Living conditions here have changed very little in the past century, and the people are not normally very mobile. Parts of South Sudan, in particular the Upper Nile region, are almost totally cut off from the rest of the country, and most people tend to remain at their place of birth although there have been huge population movements due to the civil war, leading to severe epidemics.[101]
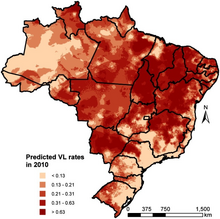
-
Geostatistical model-based predicted incidence rates per 10,000 for visceral leishmaniasis in Brazil
-
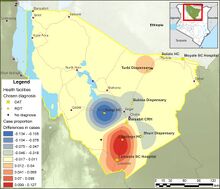
Marsabit county, Kenya-Smoothed map showing the shift in cases between 2019 and 2017 of Visceral leishmaniasis
History


Kala-azar first came to the attention of Western doctors in 1824 in Jessore, India (now Bangladesh), where it was initially thought to be a form of malaria. Assam gave kala-azar one of its common names, Assam fever.[102] Another common name, kala-azar (Hindustani: काला आज़ार (Devanagari) کالا آزار (Nastaleeq) kālā āzār), is derived from kala which means black in Sanskrit, as well as in the languages descended from it, including Assamese,[103] Hindi and Urdu;[104] the word azar means Fever in Persian and Hindustani;[103][105] as such the disease is named for the darkening of the skin on the extremities and abdomen that is a symptom of the Indian form of the disease. The agent of the disease was also first isolated in India by Scottish doctor William Leishman (who observed the parasite in spleen smears of a soldier who died of the disease in Dumdum, Calcutta, India[106] - hence the name dumdum fever) and Irish physician Charles Donovan, working independently of each other. As they published their discovery almost simultaneously, the species was named for both of them—Leishmania donovani.[107]
Today, the name kala-azar is used interchangeably with the scientific name visceral leishmaniasis for the most acute form of the disease caused by L. donovani. The disease is endemic in West Bengal, where it was first discovered, but is seen at its most deadly in north and east Africa. It can also be found throughout the Arab world and southern Europe (causative organism is L. infantum), and a slightly different strain of the pathogen, L. chagasi, is responsible for leishmaniasis in the new world. Several species of canines serve as reservoir hosts of L. infantum .[108][109][110]
Contemporary life has made itself felt even here, however—not as "progress" but in the form of the many small wars of Africa's post-colonial era. In the Sudan, where civil war has been continuous since 1983, the violence has been concentrated in the more populated south, and kala-azar was concentrated there too. But the wars have driven a steady stream of refugees out of the region, and these traveled either across the southern border or into the remoter western part of the country called the Upper Nile, where both war and the disease that went with it had not yet penetrated.[101]
These refugees, moving at foot-speed, carried the disease with them, and when it arrived it hit the Upper Nile with a force comparable to smallpox hitting the American Indians. The isolated people of the Upper Nile had no access to medicine or education about the new disease among them. Worse, their immune systems were defenseless against this new pathogen, foreign to them though it came only from another part of their own country. One village at the center of the epidemic, Duar, was left with four survivors out of a population of a thousand, and from the late eighties to the mid-nineties a total of 100,000 succumbed to the sickness in that region alone. In the words of Jill Seaman, the doctor who led relief efforts in the Upper Nile for the French organization Médecins Sans Frontières, "Where else in the world could 50% of a population die without anyone knowing?"[111] Due to the South Sudanese Civil War, kala-azar has spread rapidly among the population.[112]
The Indian medical practitioner Upendra Nath Brahmachari was nominated for the Nobel Prize in Physiology or Medicine in 1929 for his discovery of ureastibamine (an antimonial compound for the treatment of kala-azar) and a new disease, post kala-azar dermal leishmaniasis.[113]
Brahmachari's cure for visceral leishmaniasis was the urea salt of para-amino-phenyl stibnic acid which he called Urea Stibamine.[114]During the nineteenth century, kala-azar was discovered near moving bodies of water in southeast Asia.[115] Dr. Jean Dow and Dr. William McClure, are credited with finding the cure for the disease in China. Largely uncredited for her contribution, Dr. Jean Dow was one of the first to isolate the microorganism in China and conduct clinical studies on its origin.[116] This work continued under Ernest Struthers and Lionel Napier at the School of Tropical Medicine at Calcutta to discover that kala-azar was transmitted by sandflies.[117][118]
Research
Combination drug therapies are currently under investigation, particularly by the Drugs for Neglected Diseases initiative (DNDi)[119][120]. Combination therapies allow for the use of existing drugs in combination, each in lower doses, which helps to decrease the incidence of severe side effects and drug toxicity, as well as the risk for development of resistance against the drugs; they have been shown to be cost-effective strategies.[121] Comparative homology modelling of the enzyme Hypoxanthine-guanine phosphoribosyl transferase (HGPRT; EC 2.4.2.8) in L. donovani suggest that among all of the computationally screened compounds, pentamidine, 1,3-dinitroadamantane, acyclovir and analogs of acyclovir had higher binding affinities than the real substrate (guanosine monophosphate).[122]
DNDi has a number of compounds in preclinical and phase 1 development,[123] but no novel drugs are expected in the next 5 years.[124] In the meantime, new combination therapies, and well as improvements to existing drugs targets, are under development. Single-dosage administrations of liposomal amphotericin B have been shown to be effective, and oral formulations are currently under development to increase access and facilitate distribution of the efficacious drug in the field.[125][126][127]
A 2018 study published details of a new potential preclinical drug candidate for the treatment for visceral leishmaniasis with an anti-leishmanial drug-like chemical series based on a pyrazolopyrimidine scaffold.[128]There is no good vaccine candidate which prevents kala azar. A 2019 paper described designing an immunologic adjuvant which would make a VL vaccine more effective.[129]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "Leishmaniasis". www.who.int. Archived from the original on 26 July 2019. Retrieved 16 July 2023.
- ↑ 2.0 2.1 2.2 2.3 2.4 Burza, S; Croft, SL; Boelaert, M (15 September 2018). "Leishmaniasis". Lancet (London, England). 392 (10151): 951–970. doi:10.1016/S0140-6736(18)31204-2. PMID 30126638.
- ↑ Nazzaro, Gianluca; Rovaris, Marco; Veraldi, Stefano (1 November 2014). "Leishmaniasis: A Disease With Many Names". JAMA Dermatology. 150 (11): 1204. doi:10.1001/jamadermatol.2014.1015.
- ↑ 4.0 4.1 4.2 4.3 4.4 van Griensven, J; Diro, E (March 2019). "Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens". Infectious disease clinics of North America. 33 (1): 79–99. doi:10.1016/j.idc.2018.10.005. PMID 30712769.
- ↑ 5.0 5.1 "Visceral leishmaniasis — Level 4 cause". Institute for Health Metrics and Evaluation. 15 October 2020. Archived from the original on 18 July 2023. Retrieved 16 July 2023.
- ↑ "WHO guideline for the treatment of visceral leishmaniasis in HIV co-infected patients in East Africa and South-East Asia". www.who.int. Archived from the original on 11 May 2023. Retrieved 16 July 2023.
- ↑ Saha, P; Chaudhury, A; Maji, AK (July 2021). "Sir U.N. Brahmachari and his battle against Kala-Azar". Tropical Parasitology. 11 (2): 89–91. doi:10.4103/tp.tp_48_21 (inactive 2023-07-05). PMC 8579769. PMID 34765528.
{{cite journal}}: CS1 maint: DOI inactive as of July 2023 (link) - ↑ Baker, John R.; Muller, Ralph; Rollinson, David (22 August 2002). Advances in Parasitology. Elsevier. p. 18. ISBN 978-0-08-049037-3. Archived from the original on 12 July 2023. Retrieved 11 July 2023.
- ↑ "Visceral Leishmaniasis - PAHO/WHO | Pan American Health Organization". www.paho.org. Archived from the original on 29 March 2023. Retrieved 3 July 2023.
- ↑ Prevention, CDC-Centers for Disease Control and (19 May 2020). "CDC - Leishmaniasis - General Information - Frequently Asked Questions (FAQs)". www.cdc.gov. Archived from the original on 7 May 2023. Retrieved 5 July 2023.
- ↑ van Griensven, Johan; Diro, Ermias (June 2012). "Visceral Leishmaniasis". Infectious Disease Clinics of North America. 26 (2): 309–322. doi:10.1016/j.idc.2012.03.005. Archived from the original on 22 November 2022. Retrieved 10 July 2023.
- ↑ "Visceral leishmaniasis and HIV coinfection: WHO publishes new guideline with region-specific treatment recommendations". www.who.int. Archived from the original on 10 May 2023. Retrieved 14 July 2023.
- ↑ van Griensven, Johan; Mohammed, Rezika; Ritmeijer, Koert; Burza, Sakib; Diro, Ermias (April 2018). "Tuberculosis in Visceral Leishmaniasis-Human Immunodeficiency Virus Coinfection: An Evidence Gap in Improving Patient Outcomes?". Open Forum Infectious Diseases. 5 (4): ofy059. doi:10.1093/ofid/ofy059. ISSN 2328-8957. Archived from the original on 15 July 2023. Retrieved 14 July 2023.
- ↑ Trindade, Maria Angela Bianconcini; Silva, Lana Luiza da Cruz; Braz, Lucia Maria Almeida; Amato, Valdir Sabbaga; Naafs, Bernard; Sotto, Mirian Nacagami (23 November 2015). "Post-kala-azar dermal leishmaniasis and leprosy: case report and literature review". BMC Infectious Diseases. 15: 543. doi:10.1186/s12879-015-1260-x. ISSN 1471-2334. PMC 4656188. PMID 26592919.
- ↑ Burza, Sakib; Croft, Simon L.; Boelaert, Marleen (2018-09-15). "Leishmaniasis". The Lancet. 392 (10151): 951–970. doi:10.1016/S0140-6736(18)31204-2. ISSN 0140-6736. PMID 30126638. S2CID 208790410. Archived from the original on 2022-04-03. Retrieved 2022-03-21.
- ↑ Banerjee N (December 1973). "Role of I.M.A. during natural calamities and national emergencies". Journal of the Indian Medical Association. 61 (11): 477–81. PMID 4600129.
- ↑ Rathi SK, Pandhi RK, Chopra P, Khanna N (2005). "Post-kala-azar dermal leishmaniasis: a histopathological study". Indian Journal of Dermatology, Venereology and Leprology. 71 (4): 250–3. doi:10.4103/0378-6323.16616. PMID 16394433.
- ↑ Salotra P, Singh R (March 2006). "Challenges in the diagnosis of post kala-azar dermal leishmaniasis". The Indian Journal of Medical Research. 123 (3): 295–310. PMID 16778312.
- ↑ Singh N, Ramesh V, Arora VK, Bhatia A, Kubba A, Ramam M (February 1998). "Nodular post-kala-azar dermal leishmaniasis: a distinct histopathological entity". Journal of Cutaneous Pathology. 25 (2): 95–9. doi:10.1111/j.1600-0560.1998.tb01696.x. PMID 9521498. S2CID 41796506.
- ↑ Stark D, Pett S, Marriott D, Harkness J (March 2006). "Post-kala-azar dermal leishmaniasis due to Leishmania infantum in a human immunodeficiency virus type 1-infected patient". Journal of Clinical Microbiology. 44 (3): 1178–80. doi:10.1128/JCM.44.3.1178-1180.2006. PMC 1393159. PMID 16517925.
- ↑ Sharma, V. K.; Prasad, H. R. Y.; Sethuraman, G.; Khaitan, B. K. (2007). "Combination of sodium stibogluconate and rifampicin in post kala-azar dermal leishmaniasis". Indian Journal of Dermatology, Venereology and Leprology. 73 (1): 53–54. doi:10.4103/0378-6323.30657. ISSN 0973-3922. Archived from the original on 10 July 2023. Retrieved 9 July 2023.
- ↑ Chappuis F, et al. (2007). "Visceral leishmaniasis: what are the needs for diagnosis, treatment and control?" (PDF). Nature Reviews Microbiology. 5 (11): 873–82. doi:10.1038/nrmicro1748. PMID 17938629. S2CID 6963295. Archived (PDF) from the original on 2018-10-24. Retrieved 2022-03-21.
- ↑ Missawa, Nanci A.; Michalsky, Erika Monteiro; Fortes-Dias, Consuelo Latorre; Santos Dias, Edelberto (December 2010). "Lutzomyia longipalpis naturally infected by Leishmania (L.) chagasi in Várzea Grande, Mato Grosso State, Brazil, an area of intense transmission of visceral leishmaniasis". Cadernos De Saude Publica. 26 (12): 2414–2419. doi:10.1590/s0102-311x2010001200020. ISSN 1678-4464. Archived from the original on 10 July 2023. Retrieved 8 July 2023.
- ↑ Alexander, Bruce; Lopes de Carvalho, Renata; McCallum, Hamish; Pereira, Marcos Horácio (December 2002). "Role of the Domestic Chicken (Gallus gallus)in the Epidemiology of Urban Visceral Leishmaniasis in Brazil". Emerging Infectious Diseases. 8 (12): 1480–1485. doi:10.3201/eid0812.010485. PMC 2738513. PMID 12498667.
- ↑ Salomón, Oscar Daniel; Feliciangeli, María Dora; Quintana, María Gabriela; Afonso, Margarete Martins dos Santos; Rangel, Elizabeth Ferreira (23 October 2015). "Lutzomyia longipalpis urbanisation and control". Memórias do Instituto Oswaldo Cruz. 110 (7): 831–846. doi:10.1590/0074-02760150207. Archived from the original on 19 July 2022. Retrieved 13 July 2023.
- ↑ Sukla, Soumi; Roy, Syamal; Sundar, Shyam; Biswas, Subhajit (2017). "Leptomonas seymouri narna-like virus 1 and not leishmaniaviruses detected in kala-azar samples from India". Archives of Virology. 162 (12): 3827–35. doi:10.1007/s00705-017-3559-y. PMID 28939968. S2CID 31450182.
- ↑ Sacks, DL (2001). "Leishmania-sand fly interactions controlling species-specific vector competence". Cellular Microbiology. 3 (4): 189–96. doi:10.1046/j.1462-5822.2001.00115.x. PMID 11298643. S2CID 39033146. Archived from the original on 2019-12-31. Retrieved 2022-03-21.
- ↑ Ilg, T; Stierhof, YD; Wiese, M; McConville, MJ; Overath, P (1994). "Characterization of phosphoglycan-containing secretory products of Leishmania". Parasitology. 108 (Suppl): S63-71. doi:10.1017/s0031182000075739. PMID 8084657. S2CID 22659332.
- ↑ Wilson, Mary E.; Streit, Judy A. (1 September 1996). "Visceral Leishmaniasis". Gastroenterology Clinics of North America. 25 (3): 535–551. doi:10.1016/S0889-8553(05)70262-4. ISSN 0889-8553. PMID 8863039. Retrieved 5 July 2023.
- ↑ Lodge, R; Descoteaux, A (2008). Leishmania invasion and phagosome biogenesis. Subcellular Biochemistry. Vol. 47. pp. 174–81. doi:10.1007/978-0-387-78267-6_14. ISBN 978-0-387-78266-9. PMID 18512351.
- ↑ Chappuis, François; Sundar, Shyam; Hailu, Asrat; Ghalib, Hashim; Rijal, Suman; Peeling, Rosanna W.; Alvar, Jorge; Boelaert, Marleen (2007). "Visceral leishmaniasis: what are the needs for diagnosis, treatment and control?". Nature Reviews Microbiology. 5 (11): S7–S16. doi:10.1038/nrmicro1748. PMID 17938629. S2CID 6963295.
- ↑ Pulvertaft, RJ; Hoyle, GF (1960). "Stages in the life-cycle of Leishmania donovani". Transactions of the Royal Society of Tropical Medicine and Hygiene. 54 (2): 191–6. doi:10.1016/0035-9203(60)90057-2. PMID 14435316.
- ↑ Chatterjee, K.D. (2009). Parasitology (protozoology and helminthology) in relation to clinical medicine (13th ed.). New Delhi: CBC Publishers. pp. 67–72. ISBN 9788123918105.
- ↑ Pulvertaft, R.J.V.; Hoyle, G.F. (1960). "Stages in the life-cycle of Leishmania donovani". Transactions of the Royal Society of Tropical Medicine and Hygiene. 54 (2): 191–196. doi:10.1016/0035-9203(60)90057-2. PMID 14435316.
- ↑ Costa-da-Silva, Ana Caroline; Nascimento, Danielle de Oliveira; Ferreira, Jesuino R. M.; Guimarães-Pinto, Kamila; Freire-de-Lima, Leonardo; Morrot, Alexandre; Decote-Ricardo, Debora; Filardy, Alessandra Almeida; Freire-de-Lima, Celio Geraldo (31 March 2022). "Immune Responses in Leishmaniasis: An Overview". Tropical Medicine and Infectious Disease. 7 (4): 54. doi:10.3390/tropicalmed7040054. ISSN 2414-6366.
- ↑ Carvalho E, Bacellar O, Barral A, et al. (1989). "Antigen-specific Immunosuppression in Visceral Leishmaniasis Is Cell Mediated". J. Clin. Invest. 83 (3): 860–4. doi:10.1172/JCI113969. PMC 303759. PMID 2522103.
- ↑ Malla, Nancy; Mahajan, R. C. (March 2006). "Pathophysiology of visceral leishmaniasis - some recent concepts". The Indian Journal of Medical Research. 123 (3): 267–274. ISSN 0971-5916. PMID 16778309. Archived from the original on 6 July 2023. Retrieved 4 July 2023.
- ↑ Belkaid Y (2007). "Regulatory T Cells and Infection: a Dangerous Necessity" (PDF). Nature Reviews Immunology. 7 (11): 875–88. doi:10.1038/nri2189. PMID 17948021. S2CID 28127648. Archived from the original (PDF) on 2018-10-18. Retrieved 2018-10-17.
- ↑ 39.0 39.1 Rosser E, Mauri C (2015). "Regulatory B Cells: Origin, Phenotype, and Function". Immunity. 42 (4): 607–12. doi:10.1016/j.immuni.2015.04.005. PMID 25902480.
- ↑ 40.0 40.1 Rai A, Chandreshwar P, Singh A, et al. (2012). "Regulatory T Cells Suppress T Cell Activation at the Pathologic Site in Human Visceral Leishmaniasis". PLOS ONE. 7 (2): e31551. Bibcode:2012PLoSO...731551R. doi:10.1371/journal.pone.0031551. PMC 3275558. PMID 22347492.
- ↑ 41.0 41.1 41.2 Ganguly S, Mukhopadhyay D, Das N, et al. (2010). "Enhanced Lesional FoxP3 Expression and Peripheral Anergic Lymphocytes Indicate a Role for Regulatory T Cells in Indian Post-Kala-azar Dermal Leishmaniasis". J. Invest. Dermatol. 130 (4): 1013–22. doi:10.1038/jid.2009.393. PMID 20032994.
- ↑ Nylen S, Maurya R, Eidsmo L, et al. (2007). "Splenic Accumulation of IL-10 mRNA T Cells Distinct from CD4+ CD25+ (FoxP3+) Regulatory T Cells in Human Visceral Leishmaniasis". J Exp Med. 204 (4): 805–17. doi:10.1084/jem.20061141. PMC 2118563. PMID 17389235.
- ↑ 43.0 43.1 Gautam S, Kumar R, Singh N, et al. (2014). "CD8 T Cell Exhaustion in Human Visceral Leishmaniasis". J. Infect. Dis. 209 (2): 290–99. doi:10.1093/infdis/jit401. PMC 3873784. PMID 23922369.
- ↑ 44.0 44.1 44.2 Peruhype-Magalhaes V, Martins-Filho O, Prata A, et al. (2006). "Mixed Inflammatory/Regulatory Cytokine Profile Marked by Simultaneous Raise of Interferon-g and Interleukin-10 and Low Frequency of Tumor Necrosis Factor-a Monocytes Are Hallmarks of Active Human Visceral Leishmaniasis Due to Leishmania chagasi Infection". Clin. Exp. Immunol. 146 (1): 124–32. doi:10.1111/j.1365-2249.2006.03171.x. PMC 1809731. PMID 16968407.
- ↑ 45.0 45.1 45.2 Holaday B, Pompeu M, Jeronimo S, et al. (1993). "Potential Role for Interleukin-10 in the Immunosuppression Associated with Kala-azar". J. Clin. Invest. 92 (6): 2626–32. doi:10.1172/JCI116878. PMC 288459. PMID 8254019.
- ↑ 46.0 46.1 46.2 Holaday B (2000). "Role of CD8+ T Cells in Endogenous Interleukin-10 Secretion Associated with Visceral Leishmaniasis". Mem. Inst. Oswaldo Cruz. 95 (2): 217–20. doi:10.1590/s0074-02762000000200013. PMID 10733741.
- ↑ Schaut R, Lamb I, Toepp A, et al. (2016). "Regulatory IgDhi B Cells Suppress T Cell Function Via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis". J Immunol. 196 (10): 4100–9. doi:10.4049/jimmunol.1502678. PMC 4868652. PMID 27076677.
- ↑ Scarpini, S; Dondi, A; Totaro, C; Biagi, C; Melchionda, F; Zama, D; Pierantoni, L; Gennari, M; Campagna, C; Prete, A; Lanari, M (21 September 2022). "Visceral Leishmaniasis: Epidemiology, Diagnosis, and Treatment Regimens in Different Geographical Areas with a Focus on Pediatrics". Microorganisms. 10 (10): 1887. doi:10.3390/microorganisms10101887. PMID 36296164.
- ↑ Srivastava, P; Dayama, A; Mehrotra, S; Sundar, S (January 2011). "Diagnosis of visceral leishmaniasis". Transactions of the Royal Society of Tropical Medicine and Hygiene. 105 (1): 1–6. doi:10.1016/j.trstmh.2010.09.006. PMC 2999003. PMID 21074233.
- ↑ Boelaert M, Verdonck K, Menten J, et al. (June 2014). "Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease". Cochrane Database Syst Rev. 6 (6): CD009135. doi:10.1002/14651858.CD009135.pub2. PMC 4468926. PMID 24947503.
- ↑ Chappuis F, Rijal S, Soto A, Menten J, Boelaert M (2006). "A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis". Br Med J. 333 (7571): 723–6. doi:10.1136/bmj.38917.503056.7C. PMC 1592383. PMID 16882683.
- ↑ Sundar S, Singh RK, Maurya R, et al. (June 2006). "Serological diagnosis of Indian visceral leishmaniasis: direct agglutination test versus rK39 strip test". Trans. R. Soc. Trop. Med. Hyg. 100 (6): 533–7. doi:10.1016/j.trstmh.2005.08.018. PMID 16325874.
- ↑ Sundar S, Maurya R, Singh RK, et al. (January 2006). "Rapid, noninvasive diagnosis of visceral leishmaniasis in India: comparison of two immunochromatographic strip tests for detection of anti-K39 antibody". J. Clin. Microbiol. 44 (1): 251–3. doi:10.1128/JCM.44.1.251-253.2006. PMC 1351954. PMID 16390983.
- ↑ 54.0 54.1 Lockwood DN, Sundar S (October 2006). "Serological tests for visceral leishmaniasis". BMJ. 333 (7571): 711–2. doi:10.1136/bmj.38989.567083.BE. PMC 1592372. PMID 17023436.
- ↑ Pasquau F, Ena J, Sanchez R, et al. (June 2005). "Leishmaniasis as an opportunistic infection in HIV-infected patients: determinants of relapse and mortality in a collaborative study of 228 episodes in a Mediterranean region". Eur. J. Clin. Microbiol. Infect. Dis. 24 (6): 411–8. doi:10.1007/s10096-005-1342-6. PMID 15928908. S2CID 26991291.
- ↑ 56.0 56.1 56.2 "Parasites-Leishmaniasis Prevention and Control". January 10, 2013. Archived from the original on March 30, 2019. Retrieved April 29, 2014.
- ↑ Gillespie, Portia M.; Beaumier, Coreen M.; Strych, Ulrich; Hayward, Tara; Hotez, Peter J.; Bottazzi, Maria Elena (2016-06-03). "Status of vaccine research and development of vaccines for leishmaniasis". Vaccine. 34 (26): 2992–2995. doi:10.1016/j.vaccine.2015.12.071. ISSN 1873-2518. PMID 26973063.
- ↑ "Leishmaniasis, Visceral | CDC Yellow Book 2024". wwwnc.cdc.gov. Archived from the original on 22 June 2023. Retrieved 6 July 2023.
- ↑ Faber, Claudia; Quiñonez, Carlos Montenegro; Horstick, Olaf; Rahman, Kazi Mizanur; Runge-Ranzinger, Silvia (19 May 2022). "Indoor residual spraying for the control of visceral leishmaniasis: A systematic review". PLOS Neglected Tropical Diseases. 16 (5): e0010391. doi:10.1371/journal.pntd.0010391. ISSN 1935-2735. Archived from the original on 4 June 2022. Retrieved 11 July 2023.
- ↑ "DNDi Annual Report 2015" (PDF). Drugs for Neglected Diseases initiatives. Archived (PDF) from the original on 2019-07-26. Retrieved 2016-09-19.
- ↑ Sundar S, More DK, Singh MK, et al. (October 2000). "Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic". Clin. Infect. Dis. 31 (4): 1104–7. doi:10.1086/318121. PMID 11049798.
- ↑ Thakur CP, Narayan S, Ranjan A (September 2004). "Epidemiological, clinical & pharmacological study of antimony-resistant visceral leishmaniasis in Bihar, India" (PDF). Indian J. Med. Res. 120 (3): 166–72. PMID 15489554. Archived (PDF) from the original on 2019-04-03. Retrieved 2022-03-21.
- ↑ Thakur CP, Singh RK, Hassan SM, Kumar R, Narain S, Kumar A (1999). "Amphotericin B deoxycholate treatment of visceral leishmaniasis with newer modes of administration and precautions: a study of 938 cases". Trans. R. Soc. Trop. Med. Hyg. 93 (3): 319–23. doi:10.1016/S0035-9203(99)90037-8. PMID 10492770.
- ↑ Thakur CP, Pandey AK, Sinha GP, Roy S, Behbehani K, Olliaro P (1996). "Comparison of three treatment regimens with liposomal amphotericin B (AmBisome) for visceral leishmaniasis in India: a randomized dose-finding study". Trans. R. Soc. Trop. Med. Hyg. 90 (3): 319–22. doi:10.1016/S0035-9203(96)90271-0. PMID 8758093.
- ↑ Sundar S, Mehta H, Chhabra A, et al. (March 2006). "Amphotericin B colloidal dispersion for the treatment of Indian visceral leishmaniasis". Clin. Infect. Dis. 42 (5): 608–13. doi:10.1086/500138. PMID 16447104.
- ↑ New treatment for kala azar, the most deadly parasitic disease after malaria. ScienceDaily, 23 September 2011
- ↑ FDA News Release (19 March 2014). "FDA approves Impavido to treat tropical disease leishmaniasis". FDA. Archived from the original on 3 September 2014. Retrieved 21 March 2022.
- ↑ "Miltefosine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 11 August 2022. Retrieved 11 July 2023.
- ↑ Rijal, Suman; Ostyn, Bart; Uranw, Surendra; Rai, Keshav; Bhattarai, Narayan Raj; Dorlo, Thomas P. C.; Beijnen, Jos H.; Vanaerschot, Manu; Decuypere, Saskia; Dhakal, Subodh S.; Das, Murari Lal (2013-06-01). "Increasing Failure of Miltefosine in the Treatment of Kala-azar in Nepal and the Potential Role of Parasite Drug Resistance, Reinfection, or Noncompliance". Clinical Infectious Diseases. 56 (11): 1530–1538. doi:10.1093/cid/cit102. ISSN 1058-4838. PMID 23425958. Archived from the original on 2022-02-01. Retrieved 2022-03-21.
- ↑ Berman, Jonathan (July 2005). "Miltefosine to treat leishmaniasis". Expert Opinion on Pharmacotherapy. 6 (8): 1381–1388. doi:10.1517/14656566.6.8.1381. ISSN 1744-7666. Archived from the original on 18 June 2022. Retrieved 12 July 2023.
- ↑ 71.0 71.1 Das, Aritra; Karthick, Morchan; Dwivedi, Shweta; Banerjee, Indranath; Mahapatra, Tanmay; Srikantiah, Sridhar; Chaudhuri, Indrajit (2016-11-01). "Epidemiologic Correlates of Mortality among Symptomatic Visceral Leishmaniasis Cases: Findings from Situation Assessment in High Endemic Foci in India". PLOS Neglected Tropical Diseases. 10 (11): e0005150. doi:10.1371/journal.pntd.0005150. ISSN 1935-2735. PMC 5117587. PMID 27870870.
- ↑ A Small Charity Takes the Reins in Fighting a Neglected Disease Archived 2016-12-20 at the Wayback Machine, New York Times, July 31, 2006.
- ↑ NEW CURE FOR DEADLY VISCERAL LEISHMANIASIS (KALA-AZAR) APPROVED BY GOVERNMENT OF INDIA Archived 2007-07-06 at the Wayback Machine, Institute for OneWorld Health Press Release, September 8, 2006.
- ↑ Saha S, Mondal S, Banerjee, et al. (2006). "Immune Responses in Kala-azar". Indian J Med Res. 123 (3): 245–66. PMID 16778308.
- ↑ Manson-Bahr P (1961). "Immunity in Kala-azar". Trans. R. Soc. Trop. Med. Hyg. 55 (6): 550–55. doi:10.1016/0035-9203(61)90078-5. PMID 14469435.
- ↑ Manson-Bahr P (1967). "Cryptic Infections of Humans in an Endemic Kala-azar Area". E. African Med. J. 44 (4): 177–82. ISSN 0012-835X. Archived from the original on 2022-04-03. Retrieved 2022-03-21.
- ↑ 77.0 77.1 Carvalho E, Teixeira R, Johnson W Jr (1981). "Cell Mediated Immunity in American Visceral Leishmaniasis: Reversible Immunosuppression during Acute Infection". Infect. Immun. 33 (2): 498–502. doi:10.1128/IAI.33.2.498-500.1981. PMC 350726. PMID 7275314.
- ↑ 78.0 78.1 Carvalho E, Badaro R, Reed S, et al. (1985). "Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis". J. Clin. Invest. 76 (6): 2066–2069. doi:10.1172/JCI112209. PMC 424308. PMID 3935667.
- ↑ Carvalho E, Barral A, Pedral-Sampaio D, et al. (1992). "Immunologic Markers of Clinical Evolution in Children Recently Infected with Leishmania donovani chagasi". J. Infect. Dis. 165 (3): 535–40. doi:10.1093/infdis/165.3.535. PMID 1347057.
- ↑ 80.0 80.1 Carvalho E, Bacellar O, Brownell C, et al. (1994). "Restoration of IFN-gamma Production and Lymphocyte Proliferation in Visceral Leishmaniasis". J. Immunol. 15 (12): 5949–56. doi:10.4049/jimmunol.152.12.5949. PMID 8207220. S2CID 31372945.
- ↑ 81.0 81.1 Ghalib H, Whittle J, Kubin M, et al. (1995). "IL-12 Enhances Th-1 Type Responses in Human Leishmania donovani Infections". J. Immunol. 154 (9): 4623–9. doi:10.4049/jimmunol.154.9.4623. PMID 7722314. S2CID 37383454.
- ↑ Holaday B, Pompeu M, Evans T, et al. (1993). "Correlates of Leishmania-specific Immunity in the Clinical Spectrum of Infection with Leishmania chagasi". J. Infect. Dis. 167 (2): 411–17. doi:10.1093/infdis/167.2.411. PMID 8421174.
- ↑ Bacellar O, Brodskyn C, Guerreiro J, et al. (1996). "Interleukin-12 Restores Interferon-γ Production and Cytotoxic Responses in Visceral Leishmaniasis" (PDF). J. Infect. Dis. 173 (6): 1515–8. doi:10.1093/infdis/173.6.1515. PMID 8648233. Archived from the original on 2022-04-03. Retrieved 2022-03-21.
- ↑ 84.0 84.1 84.2 Holaday B (1999). "Immunotherapy for Visceral Leishmaniasis: Ability of Factors Produced during Anti-Leishmania Responses of Skin Test Positive Adults to Inhibit Peripheral Blood Mononuclear Cell Activities Associated with Visceral Leishmaniasis". Mem. Inst. Oswaldo Cruz. 94 (1): 55–66. doi:10.1590/S0074-02761999000100013. PMID 10029912.
- ↑ Bacellar O, D'oliveira A Jr, Jerônimo S, et al. (2000). "IL-10 and IL-12 Are the Main Regulatory Cytokines in Visceral Leishmaniasis". Cytokine. 12 (8): 1228–31. doi:10.1006/cyto.2000.0694. PMID 10930301.
- ↑ Kemp K, Kemp M, Kharazmi A, et al. (1999). "Leishmania-specific T Cells Expressing Interferon-gamma (IFN-γ) and IL-10 upon Activation Are Expanded in Individuals Cured of Visceral Leishmaniasis". Clin. Exp. Immunol. 116 (3): 500–4. doi:10.1046/j.1365-2249.1999.00918.x. PMC 1905302. PMID 10361241.
- ↑ 87.0 87.1 Saha S, Mondal S, Ravindran R, et al. (2007). "IL-10- and TGF-B-Mediated Susceptibility in Kala-azar and Post-kala-azar Dermal Leishmaniasis: The Significance of Amphotericin B in the Control of Leishmania donovani Infection in India". J. Immunol. 179 (8): 5592–5603. doi:10.4049/jimmunol.179.8.5592. PMID 17911647.
- ↑ Kemp M, Kurtzhals J, Bendtzen K, et al. (1993). "Leishmania donovani-reactive TH1-like T Cell Clones from Individuals Who Have Recovered from Visceral Leishmaniasis". Infect. Immun. 61 (3): 1069–73. doi:10.1128/IAI.61.3.1069-1073.1993. PMC 302840. PMID 8432588.
- ↑ 89.0 89.1 Mary C, Auriault V, Faugere B, et al. (1999). "Control of Leishmania infantum Infection Is Associated with CD8+ and Gamma-interferon and Interleukin-5 Producing CD4+ Antigen-specific T Cells". Infect. Immun. 67 (11): 5559–66. doi:10.1128/IAI.67.11.5559-5566.1999. PMC 96926. PMID 10531200.
- ↑ Ho M, Koech D, Iha D, et al. (1983). "Immune Suppression in Kenyan Visceral Leishmaniasis". Clin. Exp. Immunol. 51 (2): 207–14. PMC 1536899. PMID 6839538.
- ↑ Haldar J, Ghose S, Saha K, et al. (1983). "Cell-mediated Immune Response in Indian Kala-azar and Post-kala-azar Dermal Leishmaniasis". Infect. Immun. 42 (2): 702–7. doi:10.1128/IAI.42.2.702-707.1983. PMC 264486. PMID 6642649.
- ↑ Cillari E, Vitale G, Arcoleo F, et al. (1995). "In Vivo and In Vitro Cytokine and Mononuclear Cell Subsets in Sicilian Patients with Visceral Leishmaniasis". Cytokine. 7 (7): 740–5. doi:10.1006/cyto.1995.0088. PMID 8580385.
- ↑ Hailu A, van Baarle D, Knol G, et al. (2005). "T Cell and Cytokine Profiles in Human Visceral Leishmaniasis during Active and Asymptomatic or Sub-clinical Infection with Leishmania donovani". Clin. Immunol. 117 (2): 182–91. doi:10.1016/j.clim.2005.06.015. hdl:1874/380043. PMID 16125466. S2CID 3186886.
- ↑ Karp C, el-Safi S, Wynn T, et al. (1993). "In vivo Cytokine Profiles in Patients with Kala-azar. Marked Elevation of Both Interleukin-10 and Interferon-gamma". J. Clin. Invest. 91 (4): 1664–8. doi:10.1172/JCI116372. PMC 288142. PMID 8097208.
- ↑ Ghalib H, Piuvezam M, Skeiky Y, et al. (1993). "Interleukin-10 Production Correlates with Pathology in Human Leishmania donovani Infections". J Clin Invest. 92 (1): 324–9. doi:10.1172/JCI116570. PMC 293600. PMID 8326000.
- ↑ Medeiros I, Castelo A, Salomão R (1998). "Presence of Circulating Levels of Interferon-gamma, Interleukin-10 and Tumor Necrosis Factor-alpha in Patients with Visceral Leishmaniasis". Rev Inst Med Trop Sao Paulo. 40 (1): 31–4. doi:10.1590/S0036-46651998000100007. PMID 9713135.
- ↑ Peruhype-Magalhaes V, Martins-Filho O, Prata A, et al. (2005). "Immune Response in Human Visceral Leishmaniasis: Analysis of the Correlation between Innate Immunity Cytokine Profile and Disease Outcome". Scand. J. Immunol. 62 (5): 487–495. doi:10.1111/j.1365-3083.2005.01686.x. PMID 16305646. S2CID 25506998.
- ↑ Gasim S, Elhassan A, Khalil E, et al. (1998). "High Levels of Plasma IL-10 and Expression of IL-10 by Keratinocytes during Visceral Leishmaniasis Predict Subsequent Development of Post-kala-azar Dermal Leishmanniasis". Clin. Exp. Immunol. 111 (1): 64–9. doi:10.1046/j.1365-2249.1998.00468.x. PMC 1904865. PMID 9472662.
- ↑ Elshafie A, Ahlin E, Mathsson L, et al. (2007). "Circulating Immune Complexes (IC) and IC-induced Levels of GM-CSF Are Increased in Sudanese Patients with Acute Visceral Leishmania donovani Infection Undergoing Sodium Stibogluconate Treatment: Implications for Disease Pathogenesis". J. Immunol. 178 (8): 5383–89. doi:10.4049/jimmunol.178.8.5383. PMID 17404324.
- ↑ Hasker, E; Singh, SP; Malaviya, P; Picado, A; Gidwani, K; Singh, RP; Menten, J; Boelaert, M; Sundar, S (October 2012). "Visceral leishmaniasis in rural bihar, India". Emerging infectious diseases. 18 (10): 1662–4. doi:10.3201/eid1810.111083. PMID 23017164. Archived from the original on 2021-05-25. Retrieved 2023-07-13.
- ↑ 101.0 101.1 101.2 Jean, Francois (1995). "Sudan: Speak no Evil, Do no Good". Life, Death and Aid: The Médecins Sans Frontières Report on World Crisis Intervention.
- ↑ "Mosby's Medical Dictionary, 8th edition. © 2009, Elsevier.". "kala-azar". Archived from the original on 2011-09-11. Retrieved 2010-01-21.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ 103.0 103.1 HarperCollins Publishers, 1991, 1994, 1998, 2000, 2003. "kala-azar". Archived from the original on 2018-01-23. Retrieved 2010-01-21.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Merriam-Webster's online dictionary. "kala-azar". Archived from the original on 2017-10-23. Retrieved 2010-01-21.
- ↑ Taku, Thomas A. (1999). Framework for Industrialization in Africa. Greenwood Publishing Group. p. 77. ISBN 9780275964986. Archived from the original on 2022-04-03. Retrieved 2022-03-21.
Locally, the disease was called kala-azar or black fever, which is the meaning in the Hindustani language.
- ↑ Paniker, C.K. Jayaram (2013). Textbook of Medical Parasitology. Shorakhutte, Kathmandu, Nepal: Jaypee Brothers, Medical Publishers. p. 51. ISBN 978-93-5090-534-0. Archived from the original on 2021-01-26. Retrieved 2022-03-21.
- ↑ Dutta, Achintya Kumar (2003). "Professor J.C. Jha Prize Essay: MICROBE UNDER MICROSCOPE: CHARLES DONOVAN ON "LEISHMANIA DONOVANI"". Proceedings of the Indian History Congress. 64: 1164–1176. ISSN 2249-1937. Archived from the original on 10 July 2023. Retrieved 6 July 2023.
- ↑ Guha, Ushnish; Chatterjee, Moytrey; Sardar, Ashif Ali; Jana, Kingsuk; Saha, Pabitra; Maji, Ardhendu Kumar; Guha, Subhasish Kamal (7 December 2020). "Assessment of Knowledge, Attitudes, and Practices about Visceral Leishmaniasis in Endemic Areas of Malda District, West Bengal, India". The American Journal of Tropical Medicine and Hygiene. 104 (2): 646–652. doi:10.4269/ajtmh.20-0720. ISSN 1476-1645. Archived from the original on 6 April 2021. Retrieved 10 July 2023.
- ↑ Fernández-Arévalo, Anna; El Baidouri, Fouad; Ravel, Christophe; Ballart, Cristina; Abras, Alba; Lachaud, Laurence; Tebar, Silvia; Lami, Patrick; Pratlong, Francine; Gállego, Montserrat; Muñoz, Carme (November 2020). "The Leishmania donovani species complex: A new insight into taxonomy☆". International Journal for Parasitology. 50 (13): 1079–1088. doi:10.1016/j.ijpara.2020.06.013. ISSN 1879-0135. Archived from the original on 2023-07-15. Retrieved 2023-07-14.
- ↑ Ready, Paul (May 2014). "Epidemiology of visceral leishmaniasis". Clinical Epidemiology: 147. doi:10.2147/CLEP.S44267. Archived from the original on 11 October 2022. Retrieved 14 July 2023.
- ↑ Dowell, William (1997). "Rescue in Sudan". Time.
- ↑ "As South Sudan implodes, America reconsiders its support for the regime". The Economist. 12 October 2017. Archived from the original on 15 February 2018. Retrieved 21 March 2022.
- ↑ Nobel Foundation (2008).The Nomination Database for the Nobel Prize in Physiology or Medicine, 1901-1951 Archived 2016-05-08 at the Wayback Machine
- ↑ Upendra Nath Brahmachari: A Pioneer of Modern Medicine in India Archived 2018-12-25 at the Wayback Machine. Vigyan Prasar: Government of India
- ↑ Christensen, Erleen (2005). In War and Famine : Missionaries in China's Honan Province in the 1940s. MQUP. p. 195. ISBN 9780773572591.
- ↑ Figuring it out : science, gender, and visual culture. Shteir, Ann B., 1941-, Lightman, Bernard V., 1950- (1st ed.). Hanover, N.H.: Dartmouth College Press. 2006. ISBN 1-58465-602-6. OCLC 70673140.
{{cite book}}: CS1 maint: others (link) - ↑ "Dr. L. Everard Napier". Indian Medical Gazette. 78 (5): 252. May 1943. PMC 5158438. PMID 29012190.
- ↑ Gewurtz, Margo S. (2017-01-01). "Transnationalism in Missionary Medicine: The Case of Kala-azar in China and India, 1909–1946". Social Sciences and Missions. 30 (1–2): 30–43. doi:10.1163/18748945-03001001. ISSN 1874-8945.
- ↑ Freitas-Junior, Lucio H.; Chatelain, Eric; Kim, Helena Andrade; Siqueira-Neto, Jair L. (December 2012). "Visceral leishmaniasis treatment: What do we have, what do we need and how to deliver it?". International Journal for Parasitology: Drugs and Drug Resistance. 2: 11–19. doi:10.1016/j.ijpddr.2012.01.003. Archived from the original on 28 November 2022. Retrieved 7 July 2023.
- ↑ Sundar, Shyam; Sinha, Prabhat Kumar; Rai, Madhukar; Verma, Deepak Kumar; Nawin, Kumar; Alam, Shanawwaj; Chakravarty, Jaya; Vaillant, Michel; Verma, Neena; Pandey, Krishna; Kumari, Poonam; Lal, Chandra Shekhar; Arora, Rakesh; Sharma, Bhawna; Ellis, Sally; Strub-Wourgaft, Nathalie; Balasegaram, Manica; Olliaro, Piero; Das, Pradeep; Modabber, Farrokh (5 February 2011). "Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial". Lancet (London, England). 377 (9764): 477–486. doi:10.1016/S0140-6736(10)62050-8. ISSN 1474-547X. Archived from the original on 15 October 2022. Retrieved 7 July 2023.
- ↑ Olliaro, P., Darley, S., Laxminarayan, R.; et al. (2009). "Cost-effectiveness projections of single and combination therapies for visceral leishmaniasis in Bihar, India". Trop Med Int Health. 14 (8): 918–925. doi:10.1111/j.1365-3156.2009.02306.x. PMID 19563434. S2CID 29278489.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Ansari MY, Dikhit MR, Sahoo GC, Das P (April 2012). "Comparative modeling of HGPRT enzyme of L. donovani and binding affinities of different analogs of GMP". Int. J. Biol. Macromol. 50 (3): 637–49. doi:10.1016/j.ijbiomac.2012.01.010. PMID 22327112.
- ↑ "Portfolio – DNDi". www.dndi.org. Archived from the original on 2020-03-11. Retrieved 2020-02-13.
- ↑ den Boer, M.L., Alvar, J., Davidson, R.N.; et al. (2009). "Developments in the treatment of visceral leishmaniasis". Expert Opinion on Emerging Drugs. 14 (3): 395–410. doi:10.1517/14728210903153862. hdl:10144/127729. PMID 19708817. S2CID 32952275.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Sundar, S., Chakravarty, J., Agarwal, D.; et al. (2010). "Single-dose liposomal amphotericin B for visceral leishmaniasis in India". N Engl J Med. 362 (6): 504–512. doi:10.1056/NEJMoa0903627. PMID 20147716. S2CID 7070133. Archived from the original on 2022-04-03. Retrieved 2022-03-21.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Wasan, K. M., Wasan, E. K., Gershkovich, P.; et al. (2009). "Highly effective oral amphotericin B formulation against murine visceral leishmaniasis". J Infect Dis. 200 (3): 357–60. doi:10.1086/600105. PMID 19545212.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Arzamani K, Fazeli R, Shirzadi MR, Raeghi S, Arzamani M, Alavinia SM. Visceral Leishmaniasis in North Khorasan Province, Iran.
- ↑ Wyllie, Susan; Thomas, Michael; Patterson, Stephen; Crouch, Sabrinia; De Rycker, Manu; Lowe, Rhiannon; Gresham, Stephanie; Urbaniak, Michael D.; Otto, Thomas D. (2018-07-25). "Cyclin-dependent kinase 12 is a drug target for visceral leishmaniasis". Nature. 560 (7717): 192–197. Bibcode:2018Natur.560..192W. doi:10.1038/s41586-018-0356-z. ISSN 0028-0836. PMC 6402543. PMID 30046105.
- ↑ Ratnapriya, S; Keerti; Sahasrabuddhe, AA; Dube, A (12 June 2019). "Visceral leishmaniasis: An overview of vaccine adjuvants and their applications". Vaccine. 37 (27): 3505–3519. doi:10.1016/j.vaccine.2019.04.092. PMID 31103364. S2CID 159039859.
External links
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- CS1 maint: DOI inactive as of July 2023
- Webarchive template wayback links
- CS1 maint: multiple names: authors list
- CS1 maint: others
- Articles with hatnote templates targeting a nonexistent page
- Leishmaniasis
- Insect-borne diseases
- Parasitic infestations, stings, and bites of the skin
- RTT