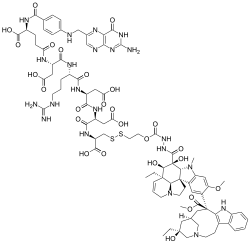
Vintafolide
 | |
| Clinical data | |
|---|---|
| Other names | EC-145 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.234.085 |
| Chemical and physical data | |
| Formula | C86H109N21O26S2 |
| Molar mass | 1917.06 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vintafolide is an investigational targeted cancer therapeutic currently under development by Endocyte and Merck & Co.[1] It is a small molecule drug conjugate consisting of a small molecule targeting the folate receptor, which is overexpressed on certain cancers, such as ovarian cancer, and a potent chemotherapy drug, vinblastine.[2]
Vintafolide is designed to deliver the toxic vinblastine drug selectively to cells expressing the folate receptor using folate targeting.[3]
It is being developed with a companion imaging agent, etarfolatide, that identifies patients that express the folate receptor and thus would likely respond to the treatment with vintafolide.[4]
A Phase 3 study evaluating vintafolide for the treatment of platinum-resistant ovarian cancer (PROCEED trial) and a Phase 2b study(TARGET trial) in non-small-cell lung carcinoma (NSCLC) are ongoing (in 2012).[5]
A Marketing Authorization Application (MAA) filing for vintafolide and etarfolatide for the treatment of patients with folate receptor-positive platinum-resistant ovarian cancer in combination with doxorubicin, pegylated liposomal doxorubicin (PLD), has been accepted by the European Medicines Agency.[6] The drug received orphan drug status in Europe in March 2012.[1] Merck & Co. acquired the development and marketing rights to this experimental cancer drug from Endocyte in April 2012.[1] Endocyte remains responsible for the development and commercialization of etarfolatide, a non-invasive companion imaging agent used to identify patients expressing the folate receptor that will likely respond to treatment with vintafolide.[5]
In 2014 Merck and Endocyte stopped a late-stage study (PROCEED) of vintafolide in treating ovarian cancer on the recommendation of a data safety monitoring board, saying that the drug failed to improve progression-free survival.[7][8]
Mechanism of action
Folate is required for cell division, and rapidly dividing cancer cells often express folate receptors in order to capture enough folate to support rapid cell growth. Elevated expression of the folate receptor occurs in many diseases, including other aggressively growing cancers and inflammatory disorders.[9] Vintafolide binds to the folate receptor and is subsequently taken up by the cell through a natural internalization process called endocytosis. Once inside the cell, vintafolide’s linker releases the chemotherapy drug which kills the cell.[4]
References
- ^ a b c Sridharan B (Apr 16, 2012). "Endocyte soars on cancer drug deal with Merck". Reuters.
- ^ Statement on a nonproprietary name adopted by the USAN Council, United States Adopted Names (USAN) Council, 6 April 2012
- ^ Dosio F, Milla P, Cattel L (December 2010). "EC-145, a folate-targeted Vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers". Current Opinion in Investigational Drugs. 11 (12): 1424–33. PMID 21154124.
- ^ a b Kuo PH (February 2013). "Companion Imaging Diagnostics for Targeted Therapies". Radiology Today. 14 (2): 32.
- ^ a b "Merck, Endocyte in Development Deal". Drug Development & Discovery magazine. 2012-04-25.
- ^ "EMA Accepts For Review MAA Filings For Vintafolide And Etarfolatide". rttnews.com. 2012-11-27.
- ^ Garde D (2014-05-02). "Merck halts study of the billion-dollar cancer drug vintafolide". Fierce Biotech. Retrieved 21 April 2015.
- ^ Merck and Endocyte Announce Independent DSMB Recommends Vintafolide PROCEED Phase 3 Trial Be Stopped for Futility Following Interim Analysis (undated)
- ^ Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP (March 2005). "Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay". Analytical Biochemistry. 338 (2): 284–93. doi:10.1016/j.ab.2004.12.026. PMID 15745749.
- Articles with short description
- Short description matches Wikidata
- Drugs with non-standard legal status
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles containing unverified chemical infoboxes
- Experimental cancer drugs
- Folates
- Small-molecule drug conjugates