Tobramycin/dexamethasone
| Combination of | |
|---|---|
| Tobramycin | Aminoglycoside antibiotic |
| Dexamethasone | Glucocorticoid |
| Names | |
| Trade names | Tobradex |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | Eye drops, topical |
| External links | |
| AHFS/Drugs.com | Professional Drug Facts |
| Legal | |
| Legal status | |
| Identifiers | |
| CAS Number |
|
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ATC code | |
Tobramycin/dexamethasone, sold under the brand name Tobradex, is a fixed-dose combination medication in the form of eye drops and eye ointment, marketed by Alcon.[2][3][4] The active ingredients are tobramycin (an antibiotic) and dexamethasone (a corticosteroid).[3] It is prescribed for the treatment of pink eye in combination with bacterial infections.[3]
Contraindications
It is contraindicated with herpetic and other viral eye infections. Other contraindications include fungal and mycobacterial infections because tobramycin is inactive against those, and the corticoid acts as an immunosuppressive agent, preventing the body's immune system from dealing with the infection. The drops are also contraindicated in patients with corneal lesions.[5][2][3][4]
Side effects
Similarly to other corticosteroid eye drops, side effects include hypersensitivity and, especially after long-term use, secondary eye infections, cataract (clouding of the eye lens) and increased intraocular pressure, leading to glaucoma. Consequently, the drug should not be applied longer than 24 days[5][6][7] without further medical evaluation.[3][4]
Interactions
Anticholinergic eye drops potentiate the risk of increased intraocular pressure. Systemic aminoglycoside antibiotics increase toxicity for ears, nerves and kidney.[5]
Society and culture
Brand names
Tobrason is a brand name in Jordan.[8][failed verification]
Cost
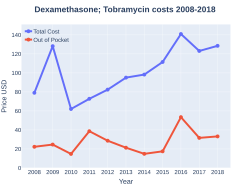
The U.S. cost for 2.5 ml ophthalmic suspension 0.1%-0.3% is $42 (USD)[9]
-
DexamethasoneTobramycin costs (US)
-
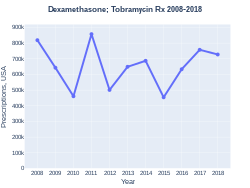
DexamethasoneTobramycin prescriptions (US)
References
- ↑ "Dexamethasone / tobramycin ophthalmic Use During Pregnancy". Drugs.com. 28 November 2018. Archived from the original on 25 November 2020. Retrieved 13 September 2020.
- ↑ 2.0 2.1 2.2 "Tobradex- tobramycin and dexamethasone ointment". DailyMed. 24 April 2020. Archived from the original on 17 August 2020. Retrieved 24 September 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Tobradex- tobramycin and dexamethasone suspension/ drops". DailyMed. 24 April 2020. Archived from the original on 17 August 2020. Retrieved 24 September 2020.
- ↑ 4.0 4.1 4.2 4.3 "Tobradex ST- tobramycin / dexamethasone suspension/ drops". DailyMed. 9 September 2019. Archived from the original on 29 October 2020. Retrieved 24 September 2020.
- ↑ 5.0 5.1 5.2 Haberfeld H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. Tobradex-Augentropfen. ISBN 978-3-85200-196-8.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Faschinger C (3 January 2011). "TobraDex". Österreichische Apothekerzeitung (in German) (1/2011): 13.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Dinnendahl V, Fricke U, eds. (2010). Arzneistoff-Profile (in German). Vol. 2 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ "Jordanian Food and Drug Administration". Archived from the original on 2020-11-16. Retrieved 2013-10-24.
- ↑ "Dexamethasone/tobramycin ophthalmic Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 January 2021. Retrieved 27 March 2021.
External links
- "Dexamethasone mixture with tobramycin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-08-29. Retrieved 2020-09-13.
- Pages using duplicate arguments in template calls
- CS1 maint: unrecognized language
- Articles with changed ChemSpider identifier
- Articles with changed KEGG identifier
- Articles without InChI source
- Multiple chemicals in Infobox drug
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Drugboxes which contain changes to verified fields
- Drugs that are a combination of chemicals
- All articles with failed verification
- Articles with failed verification from September 2020
- Articles with invalid date parameter in template
- Portal templates with all redlinked portals
- Antibiotics
- Glucocorticoids
- Combination drugs
- Novartis brands