Thiamine
 | |
 Skeletal formula and ball-and-stick model of the cation in thiamine | |
| Names | |
|---|---|
| Pronunciation | /ˈθaɪ.əmɪn/ THY-ə-min |
| Other names | Vitamin B1, aneurine, thiamin |
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | By mouth, IV, IM[2] |
| Defined daily dose | 50 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 3.7% to 5.3% |
| Chemical and physical data | |
| Formula | C12H17N4OS+ |
| Molar mass | 265.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Thiamine, also known as vitamin B1, is a vitamin found in food, and manufactured as a dietary supplement and medication.[2][4] Food sources of thiamine include whole grains, legumes, and some meats and fish.[2] Grain processing removes much of the thiamine content, so in many countries cereals and flours are enriched with thiamine.[5][2] Supplements and medications are available to treat and prevent thiamine deficiency and disorders that result from it, including beriberi and Wernicke encephalopathy.[1] Other uses include the treatment of maple syrup urine disease and Leigh syndrome.[1] They are typically taken by mouth, but may also be given by intravenous or intramuscular injection.[1][6]
Thiamine supplements are generally well tolerated.[1][7] Allergic reactions, including anaphylaxis, may occur when repeated doses are given by injection.[1][7] Thiamine is in the B complex family.[1] It is an essential micronutrient, which cannot be made in the body.[8] Thiamine is required for metabolism including that of glucose, amino acids, and lipids.[2]
Thiamine was discovered in 1897, was the first vitamin to be isolated in 1926, and was first made in 1936.[9] It is on the World Health Organization's List of Essential Medicines.[10] Thiamine is available as a generic medication, and as an over-the-counter drug.[1] The wholesale cost in the developing world is about US$2.17 per one gram vial.[11] In the United States a month's supply of a multivitamin containing thiamine is less than US$25.[12]
Medical uses
Thiamine deficiency
Thiamine is used to treat thiamine deficiency which when severe can prove fatal.[13] In less severe cases, non-specific signs include malaise, weight loss, irritability and confusion.[14] Well-known disorders caused by thiamine deficiency include beriberi, Wernicke–Korsakoff syndrome, optic neuropathy, Leigh's disease, African Seasonal Ataxia, and central pontine myelinolysis.[15]
In Western countries, thiamine deficiency is seen mainly in chronic alcoholism.[16] Thiamine deficiency is often present in alcohol misuse disorder. Also at risk are older adults, persons with HIV/AIDS or diabetes, and persons who have had bariatric surgery.[2] Varying degrees of thiamine deficiency have been associated with the long-term use of high doses of diuretics, particularly furosemide in the treatment of heart failure.[17]
-
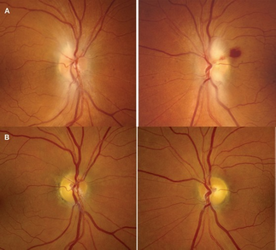
a) On initial presentation, both optic nerves had pale blurring of the disc margins superiorly and inferiorly, with peripapillary hemorrhage in the left eye. b) After thiamine tratment, one month later, the swelling had resolved, with only mild prominence of the retinal nerve fiber layer remaining bilaterally.
-
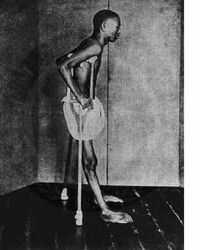
A individual with beriberi in Southeast Asia
Prenatal supplementation
Women who are pregnant or lactating require more thiamine. For pregnant and lactating women, the consequences of thiamine deficiency are the same as those of the general population but the risk is greater due to their temporarily increased need for this nutrient. In pregnancy, this is likely due to thiamine being preferentially sent to the fetus and placenta, especially during the third trimester. For lactating women, thiamine is delivered in breast milk even if it results in thiamine deficiency in the mother.[18] Pregnant women with hyperemesis gravidarum are also at an increased risk for thiamine deficiency due to losses when vomiting.[19]
Thiamine is an important aspect for not only mitochondrial membrane development, but also synaptosomal membrane function.[20] It has also been suggested that thiamine deficiency plays a role in the poor development of the infant brain that can lead to sudden infant death syndrome (SIDS).[21]
Other uses
Thiamine is a treatment for some types of maple syrup urine disease and Leigh disease.[1]
Dosage
The defined daily dose is 50 mg by mouth or by injection.[3] The dose for mild deficiency is 10 to 25 mg per day while the dose for acute beriberi is 50 mg three times per day until symptoms improved and than 10 mg once per day.[22] For beriberi in babies 10 mg once per day for three to four weeks may be used.[22]
For Wernicke’s encephalopathy or Korsakoff syndrome it may be given as 250 mg once per day by injection into a vein.[23] For beriberi it may be given at a dose of 25 to 50 mg by injection two to three times per day.[23]
Side effects
Thiamine is generally well tolerated and non-toxic when taken by mouth.[1] Rarely, side effects have been reported when thiamine is given intravenously including allergic reactions, nausea, lethargy, and impaired coordination.[24][25]
Chemistry
Thiamine is a colorless organosulfur compound with a chemical formula C12H17N4OS. Its structure consists of an aminopyrimidine and a thiazolium ring linked by a methylene bridge. The thiazole is substituted with methyl and hydroxyethyl side chains. Thiamine is soluble in water, methanol, and glycerol and practically insoluble in less polar organic solvents. It is stable at acidic pH, but is unstable in alkaline solutions.[13][26] Thiamine, which is a persistent carbene, is used by enzymes to catalyze benzoin condensations in vivo.[27] Thiamine is unstable to heat, but stable during frozen storage.[28] It is unstable when exposed to ultraviolet light[26] and gamma irradiation.[29][30] Thiamine reacts strongly in Maillard-type reactions.[13]
Biosynthesis

Complex thiamine biosynthesis occurs in bacteria, some protozoans, plants, and fungi.[31][32] The thiazole and pyrimidine moieties are biosynthesized separately and then combined to form thiamine monophosphate (ThMP) by the action of thiamine-phosphate synthase (EC2.5.1.3). The biosynthetic pathways may differ among organisms. In E. coli and other enterobacteriaceae, ThMP may be phosphorylated to the cofactor thiamine diphospate (ThDP) by a thiamine-phosphate kinase (ThMP + ATP → ThDP + ADP, EC 2.7.4.16). In most bacteria and in eukaryotes, ThMP is hydrolyzed to thiamine, which may then be pyrophosphorylated to ThDP by thiamine diphosphokinase (thiamine + ATP → ThDP + AMP, EC 2.7.6.2).
The biosynthetic pathways are regulated by riboswitches.[25] If there is sufficient thiamine present in the cell then the thiamine binds to the mRNAs for the enzymes that are required in the pathway and prevents their translation. If there is no thiamine present then there is no inhibition, and the enzymes required for the biosynthesis are produced. The specific riboswitch, the TPP riboswitch (or ThDP), is the only riboswitch identified in both eukaryotic and prokaryotic organisms.[33]
Nutrition
Occurrence in foods
Thiamine is found in a wide variety of processed and whole foods. Whole grains, legumes, pork, fruits, and yeast are rich sources.[34][35]
The salt thiamine mononitrate, rather than thiamine hydrochloride, is used for food fortification, as the mononitrate is more stable, and does not absorb water from natural humidity (is non-hygroscopic), whereas thiamine hydrochloride is hygroscopic.[citation needed] When thiamine mononitrate dissolves in water, it releases nitrate (about 19% of its weight) and is thereafter absorbed as the thiamine cation.
Dietary recommendations
| United States | ||
| Age group | RDA (mg/day) | Tolerable upper intake level[36] |
|---|---|---|
| Infants 0–6 months | 0.2* | ND |
| Infants 6–12 months | 0.3* | |
| 1–3 years | 0.5 | |
| 4–8 years | 0.6 | |
| 9–13 years | 0.9 | |
| Females 14–18 years | 1.0 | |
| Males 14+ years | 1.2 | |
| Females 19+ years | 1.1 | |
| Pregnant/lactating females 14–50 | 1.4 | |
| * Adequate intake for infants, as an RDA has yet to be established[36] | ||
| European Food Safety Authority | ||
| Age group | Adequate Intake (mg/MJ)[24] | Tolerable upper limit[24] |
| All persons 7 months+ | 0.1 | ND |
In the U.S. the Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for thiamine were updated in 1998, by the Institute of Medicine now known as the National Academy of Medicine (NAM).[36]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women (including those pregnant or lactating), men and children the PRI is 0.1 mg thiamine per megajoule (MJ) of energy consumed. As the conversion is 1 MJ = 239 kcal, an adult consuming 2390 kilocalories should be consuming 1.0 mg thiamine. This is slightly lower than the U.S. RDA.[37] The EFSA reviewed the same safety question and also reached the conclusion that there was not sufficient evidence to set a UL for thiamine.[38]
To aid with adequate micronutrient intake, pregnant women are often advised to take a daily prenatal multivitamin. While micronutrient compositions vary among different vitamins, a typical prenatal vitamin contains around 1.5 mg of thiamine.[39]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percentage of Daily Value (%DV). For thiamine labeling purposes 100% of the Daily Value was 1.5 mg, but as of May 27, 2016 it was revised to 1.2 mg to bring it into agreement with the RDA.[40][41] Compliance with the updated labeling regulations was required by 1 January 2020, for manufacturers with $10 million or more in annual food sales, and by 1 January 2021 for manufacturers with less than $10 million in annual food sales.[42][43][44] During the first six months following the 1 January 2020 compliance date, the FDA plans to work cooperatively with manufacturers to meet the new Nutrition Facts label requirements and will not focus on enforcement actions regarding these requirements during that time.[42] A table of the old and new adult Daily Values is provided at Reference Daily Intake.
Antagonists
Thiamine in foods can be degraded in a variety of ways. Sulfites, which are added to foods usually as a preservative,[45] will attack thiamine at the methylene bridge in the structure, cleaving the pyrimidine ring from the thiazole ring.[14] The rate of this reaction is increased under acidic conditions. Thiamine is degraded by thermolabile thiaminases (present in raw fish and shellfish[13]). Some thiaminases are produced by bacteria. Bacterial thiaminases are cell surface enzymes that must dissociate from the membrane before being activated; the dissociation can occur in ruminants under acidotic conditions. Rumen bacteria also reduce sulfate to sulfite, therefore high dietary intakes of sulfate can have thiamine-antagonistic activities.
Plant thiamine antagonists are heat-stable and occur as both the ortho- and para-hydroxyphenols. Some examples of these antagonists are caffeic acid, chlorogenic acid, and tannic acid. These compounds interact with the thiamine to oxidize the thiazole ring, thus rendering it unable to be absorbed. Two flavonoids, quercetin and rutin, have also been implicated as thiamine antagonists.[14]
Food fortification
Refining grain removes its bran and germ, and thus subtracts its naturally occurring vitamins and minerals. In the United States, B-vitamin deficiencies became common in the first half of the 20th century due to white flour consumption. The American Medical Association successfully lobbied for restoring these vitamins by enrichment of grain, which began in the US in 1939. The UK followed in 1940 and Denmark in 1953. As of 2016, about 85 countries had passed legislation mandating fortification of wheat flour with at least some nutrients, and 28% of industrially milled flour was fortified, often with thiamine and other B vitamins.[46]
Absorption and transport
Absorption
Thiamine is released by the action of phosphatase and pyrophosphatase in the upper small intestine. At low concentrations, the process is carrier-mediated. At higher concentrations, absorption also occurs via passive diffusion. Active transport is greatest in the jejunum and ileum, but it can be inhibited by alcohol consumption or by folate deficiency.[13] Decline in thiamine absorption occurs at intakes above 5 mg/day.[47] On the serosal side of the intestine, discharge of the vitamin by those cells is dependent on Na+-dependent ATPase.[14]
Bound to serum proteins
The majority of thiamine in serum is bound to proteins, mainly albumin. Approximately 90% of total thiamine in blood is in erythrocytes. A specific binding protein called thiamine-binding protein (TBP) has been identified in rat serum and is believed to be a hormone-regulated carrier protein important for tissue distribution of thiamine.[14]
Cellular uptake
Uptake of thiamine by cells of the blood and other tissues occurs via active transport and passive diffusion.[13] About 80% of intracellular thiamine is phosphorylated and most is bound to proteins. Two members of the SLC gene family of transporter proteins, SLC19A2 and SLC19A3, are capable of the thiamine transport.[21] In some tissues, thiamine uptake and secretion appears to be mediated by a soluble thiamine transporter that is dependent on Na+ and a transcellular proton gradient.[14]
Tissue distribution
Human storage of thiamine is about 25 to 30 mg, with the greatest concentrations in skeletal muscle, heart, brain, liver, and kidneys. ThMP and free (unphosphorylated) thiamine is present in plasma, milk, cerebrospinal fluid, and, it is presumed, all extracellular fluid. Unlike the highly phosphorylated forms of thiamine, ThMP and free thiamine are capable of crossing cell membranes. Calcium and magnesium have been shown to affect the distribution of thiamine in the body and magnesium deficiency has been shown to aggravate thiamine deficiency.[21] Thiamine contents in human tissues are less than those of other species.[14][48]
Excretion
Thiamine and its acid metabolites (2-methyl-4-amino-5-pyrimidine carboxylic acid, 4-methyl-thiazole-5-acetic acid, and thiamine acetic acid) are excreted principally in the urine.[26]
Function
Its phosphate derivatives are involved in many cellular processes. The best-characterized form is thiamine pyrophosphate (TPP), a coenzyme in the catabolism of sugars and amino acids. In yeast, TPP is also required in the first step of alcoholic fermentation. All organisms use thiamine, but it is made only in bacteria, fungi, and plants. Animals must obtain it from their diet, and thus, for humans, it is an essential nutrient. Insufficient intake in birds produces a characteristic polyneuritis.
Thiamine is usually considered as the transport form of the vitamin. There are five known natural thiamine phosphate derivatives: thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), also sometimes called thiamine pyrophosphate (TPP), thiamine triphosphate (ThTP), the recently discovered adenosine thiamine triphosphate (AThTP), and adenosine thiamine diphosphate (AThDP). While the coenzyme role of thiamine diphosphate is well-known and extensively characterized, the non-coenzyme action of thiamine and derivatives may be realized through binding to a number of recently identified proteins which do not use the catalytic action of thiamine diphosphate [49]
Thiamine diphosphate
No physiological role is known for thiamine monophosphate (ThMP); however, the diphosphate is physiologically relevant. The synthesis of thiamine diphosphate (ThDP), also known as thiamine pyrophosphate (TPP) or cocarboxylase, is catalyzed by an enzyme called thiamine diphosphokinase according to the reaction thiamine + ATP → ThDP + AMP (EC 2.7.6.2). ThDP is a coenzyme for several enzymes that catalyze the transfer of two-carbon units and in particular the dehydrogenation (decarboxylation and subsequent conjugation with coenzyme A) of 2-oxoacids (alpha-keto acids). Examples include:
- Present in most species
- Present in some species:
- pyruvate decarboxylase (in yeast)
- several additional bacterial enzymes
The enzymes transketolase, pyruvate dehydrogenase (PDH), and 2-oxoglutarate dehydrogenase (OGDH) are all important in carbohydrate metabolism. The cytosolic enzyme transketolase is a key player in the pentose phosphate pathway, a major route for the biosynthesis of the pentose sugars deoxyribose and ribose. The mitochondrial PDH and OGDH are part of biochemical pathways that result in the generation of adenosine triphosphate (ATP), which is a major form of energy for the cell. PDH links glycolysis to the citric acid cycle, while the reaction catalyzed by OGDH is a rate-limiting step in the citric acid cycle. In the nervous system, PDH is also involved in the production of acetylcholine, a neurotransmitter, and for myelin synthesis.[50]
Thiamine triphosphate
Thiamine triphosphate (ThTP) was long considered a specific neuroactive form of thiamine, playing a role in chloride channels in the neurons of mammals and other animals, although this is not completely understood.[21] However, recently it was shown that ThTP exists in bacteria, fungi, plants and animals suggesting a much more general cellular role.[51] In particular in E. coli, it seems to play a role in response to amino acid starvation.[52]
Adenosine thiamine triphosphate
Adenosine thiamine triphosphate (AThTP) or thiaminylated adenosine triphosphate has recently been discovered in Escherichia coli, where it accumulates as a result of carbon starvation.[53] In E. coli, AThTP may account for up to 20% of total thiamine. It also exists in lesser amounts in yeast, roots of higher plants and animal tissue.[54]
Adenosine thiamine diphosphate
Adenosine thiamine diphosphate (AThDP) or thiaminylated adenosine diphosphate exists in small amounts in vertebrate liver, but its role remains unknown.[54]
History
Thiamine was the first of the water-soluble vitamins to be described,[13] leading to the discovery of more essential nutrients and to the notion of vitamin.
In 1884, Takaki Kanehiro (1849–1920), a surgeon general in the Japanese navy, rejected the previous germ theory for beriberi and hypothesized that the disease was due to insufficiencies in the diet instead.[55] Switching diets on a navy ship, he discovered that replacing a diet of white rice only with one also containing barley, meat, milk, bread, and vegetables, nearly eliminated beriberi on a nine-month sea voyage. However, Takaki had added many foods to the successful diet and he incorrectly attributed the benefit to increased nitrogen intake, as vitamins were unknown substances at the time. The Navy was not convinced of the need for so expensive a program of dietary improvement, and many men continued to die of beriberi, even during the Russo-Japanese war of 1904–5. Not until 1905, after the anti-beriberi factor had been discovered in rice bran (removed by polishing into white rice) and in barley bran, was Takaki's experiment rewarded by making him a baron in the Japanese peerage system, after which he was affectionately called "Barley Baron".
The specific connection to grain was made in 1897 by Christiaan Eijkman (1858–1930), a military doctor in the Dutch Indies, who discovered that fowl fed on a diet of cooked, polished rice developed paralysis, which could be reversed by discontinuing rice polishing.[56] He attributed beriberi to the high levels of starch in rice being toxic. He believed that the toxicity was countered in a compound present in the rice polishings.[57] An associate, Gerrit Grijns (1865–1944), correctly interpreted the connection between excessive consumption of polished rice and beriberi in 1901: He concluded that rice contains an essential nutrient in the outer layers of the grain that is removed by polishing.[58] Eijkman was eventually awarded the Nobel Prize in Physiology and Medicine in 1929, because his observations led to the discovery of vitamins.
In 1910 a Japanese scientist Umetaro Suzuki first isolated the compound which he described as aberic acid. In translation from the Japanese paper in which it was claimed to be a new finding this claim was omitted.[59] In 1911 a Polish biochemist Casimir Funk isolated the antineuritic substance from rice bran (the modern thiamine) that he called a "vitamine" (on account of its containing an amino group).[60][61] However, Funk did not completely characterize its chemical structure. Dutch chemists, Barend Coenraad Petrus Jansen (1884–1962) and his closest collaborator Willem Frederik Donath (1889–1957), went on to isolate and crystallize the active agent in 1926,[62] whose structure was determined by Robert Runnels Williams (1886–1965), a US chemist, in 1934. Thiamine was named by the Williams team as "thio" or "sulfur-containing vitamin", with the term "vitamin" coming indirectly, by way of Funk, from the amine group of thiamine itself (by this time in 1936, vitamins were known to not always be amines, for example, vitamin C). Thiamine was synthesized in 1936 by the Williams group.[63]
Thiamine was first named "aneurin" (for anti-neuritic vitamin).[64] Sir Rudolph Peters, in Oxford, introduced thiamine-deprived pigeons as a model for understanding how thiamine deficiency can lead to the pathological-physiological symptoms of beriberi. Indeed, feeding the pigeons upon polished rice leads to an easily recognizable behavior of head retraction, a condition called opisthotonos. If not treated, the animals died after a few days. Administration of thiamine at the stage of opisthotonos led to a complete cure within 30 minutes. As no morphological modifications were observed in the brain of the pigeons before and after treatment with thiamine, Peters introduced the concept of a biochemical lesion.[65]
When Lohman and Schuster (1937) showed that the diphosphorylated thiamine derivative (thiamine diphosphate, ThDP) was a cofactor required for the oxydative decarboxylation of pyruvate,[66] a reaction now known to be catalyzed by pyruvate dehydrogenase, the mechanism of action of thiamine in the cellular metabolism seemed to be elucidated. At present, this view seems to be oversimplified: pyruvate dehydrogenase is only one of several enzymes requiring thiamine diphosphate as a cofactor; moreover, other thiamine phosphate derivatives have been discovered since then, and they may also contribute to the symptoms observed during thiamine deficiency. Lastly, the mechanism by which the thiamine moiety of ThDP exerts its coenzyme function by proton substitution on position 2 of the thiazole ring was elucidated by Ronald Breslow in 1958.[67]
- Some contributors to the discovery of thiamine
Society and culture
Cost
The wholesale cost in the developing world is about US$2.17 per one gram vial.[11] In the United States a month's supply of a multivitamin containing thiamine is less than US$25.[12]
-
Thiamine costs (US)
-
Thiamine prescriptions (US)
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 American Society of Health-System Pharmacists. "Thiamine Hydrochloride". Drugsite Trust (Drugs.com). Archived from the original on 9 August 2020. Retrieved 17 April 2018.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Office of Dietary Supplements - Thiamin". ods.od.nih.gov. 11 February 2016. Archived from the original on 30 December 2016. Retrieved 30 December 2016.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 26 February 2020. Retrieved 4 September 2020.
- ↑ "Thiamine: MedlinePlus Drug Information". medlineplus.gov. Archived from the original on 28 April 2018. Retrieved 30 April 2018.
- ↑ Guidelines on food fortification with micronutrients (PDF). WHO and FAO. 2006. pp. 13–14. ISBN 92-4-159401-2. Archived (PDF) from the original on 26 December 2016. Retrieved 5 May 2018.
- ↑ "Thiamine". www.drugbank.ca. Archived from the original on 29 April 2018. Retrieved 30 April 2018.
- ↑ 7.0 7.1 Kliegman, Robert M.; Stanton, Bonita (2016). Nelson Textbook of Pediatrics. Elsevier Health Sciences. p. 322. ISBN 9781455775668. Archived from the original on 24 April 2018. Retrieved 24 April 2018.
There are no cases of adverse effects of excess thiamine... A few isolated cases of puritis...
- ↑ Constable, Peter D.; Hinchcliff, Kenneth W.; Done, Stanley H.; Gruenberg, Walter (2017). Diseases of the Nervous System - Veterinary Medicine (Eleventh Edition) - 14. pp. 1155–1370. ISBN 978-0-7020-5246-0.
Thiamine (vitamin B1) is synthesized only in bacteria, fungi, and plants but is an essential nutrient for animals.
- ↑ Squires, Victor R. (2011). The Role of Food, Agriculture, Forestry and Fisheries in Human Nutrition - Volume IV. EOLSS Publications. p. 121. ISBN 9781848261952. Archived from the original on 30 December 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 11.0 11.1 "Vitamin B1". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- ↑ 12.0 12.1 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 230. ISBN 9781284057560.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Mahan LK, Escott-Stump S, eds. (2000). Krause's food, nutrition, & diet therapy (10th ed.). Philadelphia: W.B. Saunders Company. ISBN 978-0-7216-7904-4.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Combs Jr GF (2008). The Vitamins: Fundamental Aspects in Nutrition and Health (3rd ed.). Ithaca, NY: Elsevier Academic Press. ISBN 978-0-12-183493-7.
- ↑ McCandless, David (2010). Thiamine Deficiency and Associate Clinical Disorders. New York, NY: Humana Press. pp. 157–159. ISBN 978-1-60761-310-7.
- ↑ The Editors of Encyclopaedia Britannica (19 December 2017). "Beriberi". Encyclopædia Britannica. Archived from the original on 14 April 2018. Retrieved 13 April 2018.
- ↑ Katta N, Balla S, Alpert MA (July 2016). "Does Long-Term Furosemide Therapy Cause Thiamine Deficiency in Patients with Heart Failure? A Focused Review". The American Journal of Medicine. 129 (7): 753.e7–753.e11. doi:10.1016/j.amjmed.2016.01.037. PMID 26899752.
- ↑ Butterworth RF (December 2001). "Maternal thiamine deficiency: still a problem in some world communities". The American Journal of Clinical Nutrition. 74 (6): 712–3. doi:10.1093/ajcn/74.6.712. PMID 11722950.
- ↑ Oudman E, Wijnia JW, Oey M, van Dam M, Painter RC, Postma A (May 2019). "Wernicke's encephalopathy in hyperemesis gravidarum: A systematic review". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 236: 84–93. doi:10.1016/j.ejogrb.2019.03.006. PMID 30889425.
- ↑ Kloss O, Eskin NA, Suh M (April 2018). "Thiamin deficiency on fetal brain development with and without prenatal alcohol exposure". Biochemistry and Cell Biology. 96 (2): 169–177. doi:10.1139/bcb-2017-0082. hdl:1807/87775. PMID 28915355.
- ↑ 21.0 21.1 21.2 21.3 Lonsdale D (March 2006). "A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives". Evidence-Based Complementary and Alternative Medicine. 3 (1): 49–59. doi:10.1093/ecam/nek009. PMC 1375232. PMID 16550223.
- ↑ 22.0 22.1 "THIAMINE = VITAMIN B1 oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 25 August 2020.
- ↑ 23.0 23.1 "THIAMINE = VITAMIN B1 injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 4 September 2020.
- ↑ 24.0 24.1 24.2 Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006, archived (PDF) from the original on 16 March 2016
- ↑ 25.0 25.1 Bettendorff L (2020). "Thiamine". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 171–88. ISBN 978-0-323-66162-1.
- ↑ 26.0 26.1 26.2 Tanphaichitr V (1999). "Thiamin". In Shils ME, Olsen JA, Shike M, et al. (eds.). Modern Nutrition in Health and Disease (9th ed.). Baltimore: Lippincott Williams & Wilkins.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 14 February 2012. Retrieved 18 March 2011.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Vitamin B1 (Thiamine)". Medicine LibreTexts. 12 May 2017. Archived from the original on 19 February 2018. Retrieved 19 February 2018.
- ↑ Luczak M (1968). "Changes occurring in milk powder subjected to gamma rays". Zeszyty Problemowe Postepow Nauk Rolniczych. 80 (497–501).Chem Abstr 1969;71,2267g
- ↑ Syunyakova ZM, Karpova IN (1966). "The effect of γ-rays and thermal sterilization on the content of thiamine, riboflavine, nicotinic acid, and tocopherol in beef". Vop Pitan. 25 (2): 52–5. Chem Abstr 1966;65,1297b
- ↑ Webb ME, Marquet A, Mendel RR, Rébeillé F, Smith AG (October 2007). "Elucidating biosynthetic pathways for vitamins and cofactors". Natural Product Reports. 24 (5): 988–1008. doi:10.1039/b703105j. PMID 17898894.
- ↑ Begley TP, Chatterjee A, Hanes JW, Hazra A, Ealick SE (April 2008). "Cofactor biosynthesis--still yielding fascinating new biological chemistry". Current Opinion in Chemical Biology. 12 (2): 118–25. doi:10.1016/j.cbpa.2008.02.006. PMC 2677635. PMID 18314013.
- ↑ Bocobza SE, Aharoni A (October 2008). "Switching the light on plant riboswitches". Trends in Plant Science. 13 (10): 526–33. doi:10.1016/j.tplants.2008.07.004. PMID 18778966.
- ↑ "Thiamin content per 100 grams; select food subset, abridged list by food groups". United States Department of Agriculture, Agricultural Research Service, USDA Branded Food Products Database v.3.6.4.1. 17 January 2017. Archived from the original on 2 February 2017. Retrieved 27 January 2017.
- ↑ "Thiamin, Food sources". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 2013. Archived from the original on 2 February 2017. Retrieved 27 January 2017.
- ↑ 36.0 36.1 36.2 Institute of Medicine (1998). "Thiamin". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 58–86. ISBN 978-0-309-06554-2. Archived from the original on 16 July 2015. Retrieved 29 August 2017.
- ↑ "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017. Archived (PDF) from the original on 28 August 2017.
- ↑ Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006, archived (PDF) from the original on 16 March 2016
- ↑ Kominiarek MA, Rajan P (November 2016). "Nutrition Recommendations in Pregnancy and Lactation". The Medical Clinics of North America. 100 (6): 1199–1215. doi:10.1016/j.mcna.2016.06.004. PMC 5104202. PMID 27745590.
- ↑ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF). Archived (PDF) from the original on 8 August 2016.
- ↑ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ↑ 42.0 42.1 "FDA provides information about dual columns on Nutrition Facts label". U.S. Food and Drug Administration (FDA). 30 December 2019. Archived from the original on 23 January 2021. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 27 May 2016. Archived from the original on 6 May 2018. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 21 December 2018. Archived from the original on 25 December 2020. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ McGuire M, Beerman KA (2007). Nutritional Sciences: From Fundamentals to Foods. California: Thomas Wadsworth.
- ↑ Annemarie Hoogendoorn, Corey Luthringer, Ibrahim Parvanta and Greg S. Garrett (2016). "Food Fortification Global Mapping Study" (PDF). European Commission. pp. 121–128. Archived (PDF) from the original on 16 April 2018. Retrieved 15 April 2018.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Hayes KC, Hegsted DM. Toxicity of the Vitamins. In: National Research Council (U.S.). Food Protection Committee. Toxicants Occurring Naturally in Foods. 2nd ed. Washington DCL: National Academy Press; 1973.
- ↑ Bettendorff L, Mastrogiacomo F, Kish SJ, Grisar T (January 1996). "Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain". Journal of Neurochemistry. 66 (1): 250–8. doi:10.1046/j.1471-4159.1996.66010250.x. PMID 8522961.
- ↑ Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis : Scientific Reports Archived 31 July 2015 at the Wayback Machine
- ↑ Butterworth RF (2006). "Thiamin". In Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ (eds.). Modern Nutrition in Health and Disease (10th ed.). Baltimore: Lippincott Williams & Wilkins.
- ↑ Makarchikov AF, Lakaye B, Gulyai IE, Czerniecki J, Coumans B, Wins P, et al. (July 2003). "Thiamine triphosphate and thiamine triphosphatase activities: from bacteria to mammals". Cellular and Molecular Life Sciences. 60 (7): 1477–88. doi:10.1007/s00018-003-3098-4. PMID 12943234.
- ↑ Lakaye B, Wirtzfeld B, Wins P, Grisar T, Bettendorff L (April 2004). "Thiamine triphosphate, a new signal required for optimal growth of Escherichia coli during amino acid starvation". The Journal of Biological Chemistry. 279 (17): 17142–7. doi:10.1074/jbc.M313569200. PMID 14769791.
- ↑ Bettendorff L, Wirtzfeld B, Makarchikov AF, Mazzucchelli G, Frédérich M, Gigliobianco T, et al. (April 2007). "Discovery of a natural thiamine adenine nucleotide". Nature Chemical Biology. 3 (4): 211–2. doi:10.1038/nchembio867. PMID 17334376.
- ↑ 54.0 54.1 Frédérich M, Delvaux D, Gigliobianco T, Gangolf M, Dive G, Mazzucchelli G, et al. (June 2009). "Thiaminylated adenine nucleotides. Chemical synthesis, structural characterization and natural occurrence". The FEBS Journal. 276 (12): 3256–68. doi:10.1111/j.1742-4658.2009.07040.x. PMID 19438713.
- ↑ McCollum EV. A History of Nutrition. Cambridge, Massachusetts: Riverside Press, Houghton Mifflin; 1957.
- ↑ Eijkman C (1897). "Eine Beriberiähnliche Krankheit der Hühner" [A disease of chickens which is similar to beri-beri]. Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin. 148 (3): 523–532. doi:10.1007/BF01937576. Archived from the original on 9 August 2020. Retrieved 4 July 2019.
- ↑ "The Nobel Prize and the Discovery of Vitamins". www.nobelprize.org. Archived from the original on 16 January 2018. Retrieved 1 May 2018.
- ↑ Grijns G (1901). "Over polyneuritis gallinarum" [On polyneuritis gallinarum]. Geneeskundig Tijdschrift voor Nederlandsch-Indië (Medical Journal for the Dutch East Indies). 41 (1): 3–110. Archived from the original on 29 August 2021. Retrieved 5 February 2020.
- ↑ Suzuki U, Shimamura T (1911). "Active constituent of rice grits preventing bird polyneuritis". Tokyo Kagaku Kaishi. 32: 4–7, 144–146, 335–358. doi:10.1246/nikkashi1880.32.4. Archived from the original on 21 June 2020. Retrieved 2 May 2018.
- ↑ Funk, Casimir (1911). "On the chemical nature of the substance which cures polyneuritis in birds induced by a diet of polished rice". The Journal of Physiology. 43 (5): 395–400. doi:10.1113/jphysiol.1911.sp001481. PMC 1512869. PMID 16993097. Archived from the original on 27 April 2021. Retrieved 5 February 2020.
- ↑ Funk, Casimir (1912). "The etiology of the deficiency diseases. Beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra". Journal of State Medicine. 20: 341–368. Archived from the original on 4 July 2020. Retrieved 5 February 2020. The word "vitamine" is coined on p. 342: "It is now known that all these diseases, with the exception of pellagra, can be prevented and cured by the addition of certain preventative substances; the deficient substances, which are of the nature of organic bases, we will call "vitamines"; and we will speak of a beri-beri or scurvy vitamine, which means a substance preventing the special disease."
- ↑ Jansen BC, Donath WF (1926). "On the isolation of antiberiberi vitamin". Proc. Kon. Ned. Akad. Wet. 29: 1390–1400.
- ↑ Williams RR, Cline JK (1936). "Synthesis of vitamin B1". J. Am. Chem. Soc. 58 (8): 1504–1505. doi:10.1021/ja01299a505.
- ↑ Carpenter KJ (2000). "Beriberi, white rice, and vitamin B: a disease, a cause, and a cure". Berkeley, CA: University of California Press.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Peters RA (1936). "The biochemical lesion in vitamin B1 deficiency. Application of modern biochemical analysis in its diagnosis". Lancet. 230 (5882): 1161–1164. doi:10.1016/S0140-6736(01)28025-8.
- ↑ Lohmann K, Schuster P (1937). "Untersuchungen über die Cocarboxylase". Biochem. Z. 294: 188–214.
- ↑ Breslow R (1958). "On the mechanism of thiamine action. IV.1 Evidence from studies on model systems". J Am Chem Soc. 80 (14): 3719–3726. doi:10.1021/ja01547a064.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: archived copy as title
- Wikipedia articles incorporating the PD-notice template
- CS1 maint: multiple names: authors list
- Webarchive template wayback links
- CS1 errors: missing periodical
- Articles with hatnote templates targeting a nonexistent page
- Use dmy dates from July 2012
- Articles with invalid date parameter in template
- Chem-molar-mass both hardcoded and calculated
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from January 2017
- Thiamine
- Aminopyrimidines
- B vitamins
- Coenzymes
- Poultry diseases
- Primary alcohols
- Thiazoles
- World Health Organization essential medicines
- RTT