Tebentafusp
| Names | |
|---|---|
| Trade names | Kimmtrak |
| Other names | IMCgp100, tebentafusp-tebn |
| Clinical data | |
| Drug class | Bispecific gp100 peptide-HLA-directed CD3 T cell engager[1] |
| Main uses | Uveal melanoma[1] |
| Side effects | Cytokine release syndrome, rash, fever, itching, tiredness, nausea, abdominal pain, swelling, low blood pressure, dry skin, headache, vomiting[1] |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Typical dose | 20 to 68 mcg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
Tebentafusp, sold under the brand name Kimmtrak, is an medication used to treat a type of eye cancer, known as uveal melanoma.[1] It is used in HLA-A*02:01-positive cases that cannot be managed by surgery alone.[1][4] It is given by injection into a vein.[1] Due to the risk of side effects nearly a day of monitoring is required after the initial doses.[1]
Common side effects include cytokine release syndrome, rash, fever, itching, tiredness, nausea, abdominal pain, swelling, low blood pressure, dry skin, headache, and vomiting.[1] Use in pregnancy may harm the baby.[1] It is a bispecific gp100 peptide-HLA-directed CD3 T cell engager.[1]
Tebentafusp was approved for medical use in the United States and Europe in 2022.[1][4] In the United States the medication costs about 18,800 USD per dose.[6] While available in the United Kingdom, the cost is yet to be determined.[6]
Medical uses
Tebentafusp is indicated for HLA-A*02:01-positive adults with unresectable or metastatic uveal melanoma.[1][5][7]
It improves typical survival over standard treatment from 16 months to to 22 months.[4]
Dosage
The initial dose is typically 20 mcg, followed by 30 mcg on day 8 and 68 mcg on day 15 followed by 68 mcg weekly after that.[1]
Mechanism of action
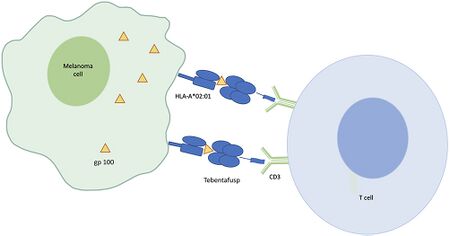
In terms of the process we find that Tebentafusp both targets and engages in its mechanism of action. It has a T-cell receptor binding domain that recognizes gp100, a melanoma-associated antigen; additionally, it has an anti-CD3 domain that redirects T cells to kill gp100-expressing tumor cells [8]
History
Efficacy was evaluated in IMCgp100-202 (NCT03070392), a randomized, open-label, multicenter trial of 378 participants with metastatic uveal melanoma.[5] Participants were required to be HLA-A*02:01 genotype positive identified by a central assay.[5] Participants were excluded if prior systemic therapy or localized liver-directed therapy were administered.[5] Prior surgical resection of oligometastatic disease was permitted.[5] Participants with clinically significant cardiac disease or symptomatic, untreated brain metastases were excluded.[5]
The U.S. Food and Drug Administration (FDA) granted Immunocore's application for tebentafusp priority review, breakthrough therapy, and orphan drug designations.[5]
Society and culture
Legal status
On 24 February 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Kimmtrak, intended for the treatment of uveal melanoma.[9][10] The applicant for this medicinal product is Immunocore Ireland Limited.[9][10] Tebentafusp was approved for medical use in the European Union in April 2022.[4]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "Kimmtrak- tebentafusp injection, solution, concentrate". DailyMed. 26 January 2022. Archived from the original on 21 February 2022. Retrieved 20 February 2022.
- ↑ 2.0 2.1 "Kimmtrak". Therapeutic Goods Administration (TGA). 15 June 2022. Archived from the original on 18 June 2022. Retrieved 18 June 2022.
- ↑ "Kimmtrak Product information". Health Canada. 25 April 2012. Archived from the original on 1 October 2022. Retrieved 30 September 2022.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Kimmtrak EPAR". European Medicines Agency (EMA). 24 January 2022. Archived from the original on 22 April 2022. Retrieved 22 April 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 "FDA approves tebentafusp-tebn for unresectable". U.S. Food and Drug Administration (FDA). 25 January 2022. Archived from the original on 27 January 2022. Retrieved 28 January 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 6.0 6.1 "Tebentafusp". SPS - Specialist Pharmacy Service. 17 September 2019. Archived from the original on 19 October 2021. Retrieved 11 December 2022.
- ↑ Damato BE, Dukes J, Goodall H, Carvajal RD (July 2019). "Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma". Cancers. 11 (7): 971. doi:10.3390/cancers11070971. PMC 6679206. PMID 31336704.
- ↑ 8.0 8.1 Montazeri, Kamaneh; Pattanayak, Vikram; Sullivan, Ryan J. (7 February 2023). "Tebentafusp in the Treatment of Metastatic Uveal Melanoma: Patient Selection and Special Considerations". Drug Design, Development and Therapy. 17: 333–339. doi:10.2147/DDDT.S368954. Archived from the original on 15 August 2023. Retrieved 4 April 2024.
- ↑ 9.0 9.1 "Kimmtrak: Pending EC decision". European Medicines Agency. 24 February 2022. Archived from the original on 26 February 2022. Retrieved 27 February 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 10.0 10.1 "New medicine for rare type of eye cancer". European Medicines Agency (EMA) (Press release). 25 February 2022. Archived from the original on 27 February 2022. Retrieved 27 February 2022.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Clinical trial number NCT03070392 for "Safety and Efficacy of IMCgp100 Versus Investigator Choice in Advanced Uveal Melanoma" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles without a structure image
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs not assigned an ATC code
- Antineoplastic drugs
- Breakthrough therapy
- Orphan drugs
- RTT