Sodium methoxide
| |
| Names | |
|---|---|
| IUPAC name
Sodium methoxide
| |
| Other names
Sodium methylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.004.273 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3NaO | |
| Molar mass | 54.02 g/mol |
| Appearance | White solid |
| Melting point | 127 °C (261 °F; 400 K) |
| Boiling point | 350 °C (662 °F; 623 K)[1] (decomposition) |
| Reacts with water | |
| Solubility | Soluble in ethanol, methanol Insoluble in hydrocarbons |
| Structure | |
| Hexagonal | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H251, H302, H314[2] | |
| P235+P410, P280, P305+P351+P338, P310[2] | |
| Safety data sheet (SDS) | Sigma[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium methoxide is the simplest sodium alkoxide. With the formula CH3ONa, it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
Preparation and structure
Sodium methoxide is prepared by treating methanol with sodium:
- 2 Na + 2 CH3OH → 2 CH3ONa + H2
The reaction is so exothermic that ignition is possible. The resulting solution, which is colorless, is often used as a source of sodium methoxide, but the pure material can be isolated by evaporation followed by heating to remove residual methanol.
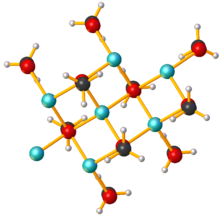
As a solid, sodium methoxide is polymeric, with sheet-like arrays of Na+ centers, each bonded to four oxygen centers.[3]
The structure, and hence the basicity, of sodium methoxide in solution depends on the solvent. It is a significantly stronger base in DMSO where it is more fully ionized and free of hydrogen bonding.[4]
Reaction Mechanism
- Brønsted Base Properties:
The high basicity of sodium methoxide is primarily due to the ability of the methoxide anion (CH3O-) to readily accept a proton, forming methanol (CH3OH) and the hydroxide ion (OH-).[5] This proton transfer reaction can be represented as follows:
CH3O- + H+ → CH3OH
The methoxide anion is a strong base, with a pKa value around 15-16, making it a potent proton acceptor1. This Brønsted base reactivity is the foundation for many of the synthetic applications of sodium methoxide, as it can be used to deprotonate acidic protons on a wide range of organic substrates. [6]
When sodium methoxide is dissolved in protic solvents like water or alcohols, the methoxide anion can undergo this proton transfer equilibrium, generating the corresponding alcohol (e.g., methanol) and a hydroxide ion. This equilibrium can be influenced by factors such as pH and the presence of other species in the solution.
- Nucleophilic Reactivity:
In addition to its Brønsted base properties, sodium methoxide can also act as a nucleophile, with the methoxide group (CH3O-) attacking electrophilic carbon centers 12.[7] This nucleophilic reactivity is particularly useful in various organic reactions, such as alkylation and esterification.
In alkylation reactions, the methoxide anion can displace a leaving group (e.g., a halide) from an alkyl halide, forming a new carbon-oxygen bond and producing an ether. This substitution reaction can be represented as follows:
R-X + CH3O- → R-O-CH3 + X-
where R represents an alkyl group and X is the leaving group (e.g., a halide).
In esterification reactions, the methoxide anion can act as a nucleophile, attacking the carbonyl carbon of an acyl halide or an anhydride, displacing the leaving group and forming a new ester bond. This process can be depicted as:
R-C(O)-X + CH3O- → R-C(O)-O-CH3 + X-
where R represents an alkyl or aryl group, and X is the leaving group (e.g., a halide or alkoxide).
The dual reactivity of sodium methoxide, both as a Brønsted base and as a nucleophile, makes it a versatile and valuable reagent in organic synthesis. The ability to deprotonate acidic protons and to undergo substitution or addition reactions with electrophilic centers allows for a wide range of synthetic transformations, including alkylations, esterifications, and other important organic reactions.[8][9]
Applications
Organic synthesis
Sodium methoxide is a routinely used base in organic chemistry, applicable to the synthesis of numerous compounds ranging from pharmaceuticals to agrichemicals.[4] As a base, it is employed in dehydrohalogenations and various condensations.[10] It is also a nucleophile for the production of methyl ethers.[11]
Industrial applications
Sodium methoxide is used as an initiator of anionic addition polymerization with ethylene oxide, forming a polyether with high molecular weight.[12] Biodiesel is prepared from vegetable oils and animal fats (fatty acid triglycerides) by transesterification with methanol to give fatty acid methyl esters (FAMEs). Sodium methoxide acts as a catalyst for this reaction, but will combine with any free fatty acids present in the oil/fat feedstock to form soap byproducts.[13]
Pharmaceuticals and Fine Chemical Synthesis
Sodium methoxide is employed in the synthesis of various pharmaceutical intermediates and fine chemicals, where its reactivity and ability to form new carbon-oxygen bonds are exploited. The methoxide group can be introduced into organic molecules through alkylation or esterification reactions, providing access to a wide range of functionalized compounds. These intermediates are then further transformed into the desired pharmaceutical active ingredients or fine chemicals through subsequent steps in the synthetic sequence.[14]
Drying Agent
Sodium methoxide can be used as a drying agent in organic synthesis, taking advantage of its ability to react with water and form methanol and sodium hydroxide. When added to organic solvents or reaction mixtures, sodium methoxide can effectively remove trace amounts of water, helping to maintain anhydrous conditions for sensitive reactions. The formation of methanol and sodium hydroxide as the reaction products makes sodium methoxide a useful alternative to other drying agents, such as magnesium sulphate or sodium sulphate.[15]
Stability
The solid hydrolyzes in water to give methanol and sodium hydroxide. Indeed, samples of sodium methoxide are often contaminated with sodium hydroxide, which is difficult to detect. The compound absorbs carbon dioxide from the air to form methanol and sodium carbonate, thus diminishing the alkalinity of the base.[16]
- CH3ONa + CO2 + H2O → 2 CH3OH + Na2CO3
Commercial batches of sodium methoxide show variable levels of degradation, and were a major source of irreproducibility when used in Suzuki reactions.[17]
Safety
Sodium methoxide is highly caustic and reacts with water to give methanol, which is toxic and volatile.
NFPA 704
The ratings for this substance vary widely.
| Rating | |||||
|---|---|---|---|---|---|
| Source | State of Connecticut[18] | DuPont[19] | Pharmco AAPR[20] | ScienceLab[21] (Both ratings on same sheet) | |
See also
References
- ^ Chandran, K.; Kamruddin, M.; Ajikumar, P.K.; et al. (2006). "Kinetics of thermal decomposition of sodium methoxide and ethoxide". Journal of Nuclear Materials. 358 (2–3): 111–128. Bibcode:2006JNuM..358..111C. doi:10.1016/j.jnucmat.2006.07.003. ISSN 0022-3115.
- ^ a b c Sigma-Aldrich Co., Sodium methoxide. Retrieved on 2022-03-21.
- ^ Weiss, E. (1964). "Die Kristallstruktur des Natriummethylats" [The Crystal Structure of Sodium Methylate]. Zeitschrift für Anorganische und Allgemeine Chemie (in German). 332 (3–4): 197–203. doi:10.1002/zaac.19643320311.
- ^ a b El-Kattan, Y.; McAtee, J.; Bessieres, B. (2006). "Sodium Methoxide". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.rs089m.pub2. ISBN 0471936235.
- ^ "Supplemental Information 1: UMP Chapter from Carvalho (2011) doctoral dissertation". dx.doi.org. Retrieved 2024-04-19.
- ^ "Novel Bifunctional Chiral Brønsted Acid/Base Organocatalysis". Synfacts. 2010 (07): 0829–0829. 2010-06-22. doi:10.1055/s-0029-1220123. ISSN 1861-1958.
- ^ Terrier, François (2013-06-03). Modern Nucleophilic Aromatic Substitution. Wiley. ISBN 978-3-527-31861-2.
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012-03-15). Organic Chemistry. Oxford University Press. ISBN 978-0-19-927029-3.
- ^ Johnson, Sasha; Meyers, Megan; Hyme, Samantha; Leontyev, Alexey (2019-12-18). "Green Chemistry Coverage in Organic Chemistry Textbooks". Journal of Chemical Education. 97 (2): 383–389. doi:10.1021/acs.jchemed.9b00397. ISSN 0021-9584.
- ^ Curtis, O. E. Jr.; Sandri, J. M.; Crocker, R. E.; Hart, H. (1958). "Dicyclopropyl ketone". Organic Syntheses. 38: 19. doi:10.15227/orgsyn.038.0019; Collected Volumes, vol. 4, p. 278.
- ^ Reverdin, F. (1927). "3,5-Dinitroanisole". Organic Syntheses. 7: 28. doi:10.15227/orgsyn.007.0028; Collected Volumes, vol. 1, p. 219.
- ^ Bailey, Frederick E.; Koleske, Joseph V. (2000). "Polyoxyalkylenes". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_579. ISBN 3527306730.
- ^ Xu, Xianyan; Kelsey, Kathleen (2012-12-01). "Will eXtension Survive? Oklahoma Cooperative Extension Service Employees' Perceptions of Adopter Attributes of eXtension". Journal of Extension. 50 (6). doi:10.34068/joe.50.06.19. ISSN 1077-5315.
- ^ Wuts, Peter G. M.; Greene, Theodora W. (2006-04-10). Greene's Protective Groups in Organic Synthesis. Wiley. ISBN 978-0-471-69754-1.
- ^ Armarego, Wilfred L.F. (2022), "Purification of Organic Chemicals", Purification of Laboratory Chemicals, Elsevier, pp. 106–595, retrieved 2024-04-19
- ^ Han, Sang-Jun; Wee, Jung-Ho (2017-01-20). "Carbon Dioxide Fixation by Combined Method of Physical Absorption and Carbonation in NaOH-Dissolved Methanol". Energy & Fuels. 31 (2): 1747–1755. doi:10.1021/acs.energyfuels.6b02709. ISSN 0887-0624.
- ^ Wethman, Robert; Derosa, Joseph; Tran, Van; et al. (2020-08-19), An Under-Appreciated Source of Reproducibility Issues in Cross-Coupling: Solid-State Decomposition of Primary Sodium Alkoxides in Air, American Chemical Society, doi:10.26434/chemrxiv.12818234.v1, S2CID 242420220
- ^ "Biodiesel: From the Field to the End User" (PDF). ct.gov. Office of Education and Data Management, State of Connecticut. 2008-04-08. Archived from the original on 2014-02-25. Retrieved 2022-01-29.
{{cite web}}: CS1 maint: unfit URL (link) - ^ "Product Safety Summary Sheet DuPont™ Sodium Methoxide (Sodium Methylate)". DuPont. 2012-09-11. Retrieved 2022-01-29 – via nanopdf.com.
- ^ "Pharmco AAPR Material Safety Data Sheet" (PDF). pharmcoaaper.com. Archived from the original (PDF) on 2014-02-23. Retrieved 2022-01-29.
- ^ "ScienceLab Material Safety Data Sheet". Archived from the original on 2015-09-09. Retrieved 2022-01-29.
- CS1 German-language sources (de)
- CS1 maint: unfit URL
- Articles with short description
- Short description is different from Wikidata
- Articles without EBI source
- Articles without KEGG source
- ECHA InfoCard ID from Wikidata
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description matches Wikidata
- Alkoxides
- Organic sodium salts
- Organic compounds with 1 carbon atom
