Infliximab
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric (mouse/human) |
| Target | TNF |
| Names | |
| Trade names | Remicade, Remsima, Inflectra, others |
| Other names | Infliximab-abda, infliximab-axxq, infliximab-dyyb, infliximab-qbtx |
| Clinical data | |
| Drug class | DMARD (biologic type)[1] |
| Main uses | Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, Behçet's disease[1] |
| Side effects | Infections, acute infusion reactions, liver problems, abdominal pain[1] |
| Pregnancy category | |
| Routes of use | Intravenous (IV) |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 92% (IV, if 8% left in the syringe) |
| Metabolism | reticuloendothelial system |
| Elimination half-life | 9.5 days |
| Chemical and physical data | |
| Formula | C6428H9912N1694O1987S46 |
| Molar mass | 144190.64 g·mol−1 |
Infliximab, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases including Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease.[1] It may be used together with methotrexate.[3] It is given by slow injection into a vein over at least 2 hours, with doses at six- to eight-week intervals.[1]
Common side effects include infections, acute infusion reactions, liver problems, and abdominal pain.[1] Other side effects may include cancer, reactivation of hepatitis B, worsening heart failure, and a lupus like condition.[1] It is unclear if use during pregnancy is safe.[4] Use during breastfeeding is likely safe for the baby.[3] Infliximab is a chimeric monoclonal antibody and biologic agent.[1] It seems to work by binding to and neutralizing TNF-α, preventing it from interacting with its receptors on the cell.[1]
Infliximab was approved for medical use in the United States in 1998,[1] and the European Union in August 1999.[5] It is on the World Health Organization's List of Essential Medicines as an alternative to adalimumab.[6] Biosimilars were approved in the EU in 2013,[7] Japan in 2014,[8] and the United States in 2016.[9] In the United Kingdom it costs about 377 pounds per 100 mg as of 2020.[3] In the United States this amount costs about 500 USD as of 2020 for a biosimilar.[10] In Canada the biosimilar is nearly half the price.[11]
Medical uses
Crohn's disease
Three categories of disease are present in Crohn's disease: stricturing disease (which causes narrowing of the bowel), penetrating disease (which causes fistulae or abnormal connections of the bowel), and inflammatory disease (which primarily causes inflammation).[12]
Fistulizing disease
Infliximab was first used for closure of fistulae in Crohn's disease in 1999. In a 94-patient, phase II clinical trial, the researchers showed Infliximab was effective in closing fistulae between the skin and bowel in 56-68% of people.[13] A large, 296-person Phase III clinical trial called the ACCENT 2 trial showed infliximab was additionally beneficial in maintaining closure of fistulae, with almost two-thirds of all patients treated with the three initial doses of infliximab having a fistula response after 14 weeks, and 36% of patients maintaining closure of fistulae after a year, compared with 19% who received placebo therapy. This final trial resulted in the FDA approval of the drug to treat fistulizing disease.[14]
Inflammatory disease
Infliximab has been used to induce and maintain remission in inflammatory Crohn's disease. The ACCENT 1 trial, a large, multicentre trial, found 39 to 45% of patients treated with infliximab, who had an initial response to it, maintained remission after 30 weeks, compared with 21% who received placebo treatment. It also showed a mean maintenance of remission from 38 to 54 weeks compared with 21 weeks for patients who received placebo treatment.[15]
Crohn's patients have flares of their disease between periods of disease quiescence. Severe flares are usually treated with steroid medications to obtain remission, but steroids have many undesirable side effects, so some gastroenterologists are now advocating the use of infliximab as the first drug to try to get patients into remission. This has been called the top-down approach to treatment.[16]
Ulcerative colitis
Infliximab targets TNF, thought to be more related to Th1 cytokines. Ulcerative colitis was thought to be a Th2 disease, and infliximab would be of limited use. However, patients with ulcerative colitis have begun to be treated with infliximab on the basis of two large clinical trials conducted in 2005 by Paul Rutgeerts and William Sandborn. The Acute ulcerative Colitis Treatment trials (ACT1 and ACT2) to evaluate the utility of infliximab in ulcerative colitis showed 44-45% of patients treated with infliximab for a year maintained a response to the medication, compared with 21% of patients who were treated with placebo medication. At two months, the response was 61-69% for patients treated with infliximab, and 31% for those treated with placebo.[17]
Newer drugs such as Etrolizumab have similar efficacy to Infliximab with less adverse effects for treatment of ulcerative colitis.[18]
Psoriatic arthritis
In psoriatic arthritis (PsA), inhibitors of TNF, such as infliximab, improve symptoms. Several therapies with modest efficacy have been studied in nail psoriasis. Among available agents, higher quality data are available to support the efficacy of cyclosporine and infliximab. Based on studies in AS, the results suggest infliximab, etanercept, and adalimumab have the potential to reduce the signs and symptoms of moderate to severely active axial involvement in PsA in patients who have had an inadequate response to NSAID (level 1a, grade A). The anti-TNF agents (infliximab and etanercept; level 1b, grade A) are more effective for the treatment of enthesitis than traditional agents. Results suggest infliximab is effective for the treatment of dactylitis in PsA.[19]
Other
It was approved for treating ankylosing spondylitis,[20] psoriatic arthritis, psoriasis, rheumatoid arthritis.[21]
Infliximab is also prescribed (out of indication) for the treatment of Behçet's disease.[22]
Infliximab is the most frequently used biological agent in treating relapsing polychondritis.[23] Half of the patients saw benefit from this treatment, and a few other patients experienced infections that in some cases lead to death.[23][24]
There have been numerous case reports of the efficacy of infliximab in various inflammatory skin conditions diseases; the FDA approved infliximab for chronic severe plaque psoriasis in adults in September 2006.[25]
Infliximab has been used off-label in treating refractory sarcoidosis, where other treatments have not been effective.[26]
Infliximab has been tested in chronic obstructive pulmonary disease (COPD) but there was no evidence of benefit with the possibility of harm.[27]
Infliximab is indicated for steroid refractory checkpoint inhibitor induced colitis, at a dose of 5 to 10 mg/kg.[28]
Dosage
The typical dose is 3 mg/kg to 5 mg/kg.[3] The initial three doses are generally given closer together.[3] Longer term doses are every six- to eight-week.[1]
Side effects
Infliximab has side effects, some life-threatening, common to drugs in the class of TNF inhibiting immunosuppressants (which also includes etanercept (Enbrel) and adalimumab (Humira)). Some of the most severe are:
- serious infections
- reactivation of hepatitis B
- reactivation of tuberculosis[29]
- lethal hepatosplenic T-cell lymphoma (generally only when combined with 6-mercaptopurine)
- drug-induced lupus
- demyelinating central nervous system disorders
- psoriasis and psoriasiform skin lesions
- new-onset vitiligo
Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia (some fatal) have been reported with infliximab.[30] The FDA issued a warning to doctors appearing in the respective product labeling of infliximab instructing them to screen and monitor potential patients more carefully.[31] The FDA issued a warning to doctors that there is an increased risk of lymphoma and other cancers associated with the use of infliximab and other tumor necrosis factor blockers in children and adolescents.[32]
Maintenance therapy with the drug (versus intermittent or sporadic therapy) lessens the likelihood of developing antibodies to infliximab which are known to reduce the efficacy of the drug. Combination treatment with methotrexate (an antifolate drug which suppresses the immune system) has been shown to reduce the formation of these antibodies in patients with rheumatoid arthritis[33] and combination therapy with other immunosuppressants has been shown to reduce the likelihood of these antibodies being formed in Crohn's disease.[15] The use of immunosuppressants may not be necessary in all diseases for which infliximab is indicated, and indiscriminant uses of these other immunosuppressants carry their own risks. Infliximab was studied in monotherapy (without concomitant immunosuppressants such as methotrexate or azathioprine) in psoriasis, psoriatic arthritis, and ankylosing spondylitis.[20] Only its use in rheumatoid arthritis requires the concomitant use of methotrexate by the U.S. Food and Drug Administration (FDA) product labeling; however, the concomitant use of methotrexate in other disease states may help to reduce the body's immune response to the infliximab and increase its duration of efficacy.[medical citation needed]
Pharmacology
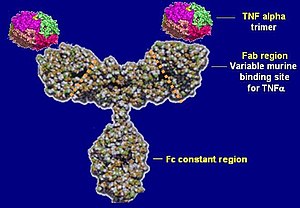
Infliximab is a purified, recombinant DNA-derived chimeric human-mouse IgG monoclonal antibody that consists of mouse heavy and light chain variable regions combined with human heavy and light chain constant regions.[34] It has a serum half-life of 9.5 days and can be detected in serum 8 weeks after infusion treatment.[34]
Infliximab neutralizes the biological activity of TNF-α by binding with high affinity to the soluble (free floating in the blood) and transmembrane (located on the outer membranes of T cells and similar immune cells) forms of TNF-α, and inhibits or prevents the effective binding of TNF-α with its receptors. Infliximab and adalimumab (another TNF antagonist) are in the subclass of "anti-TNF antibodies" (they are in the form of naturally occurring antibodies), and are capable of neutralizing all forms (extracellular-, transmembrane-, and receptor-bound) TNF-α.[35] Etanercept, a third TNF antagonist, is in a different subclass (receptor-construct fusion protein), and, because of its modified form, cannot neutralize receptor-bound TNF-α.[36] Additionally, the anti-TNF antibodies adalimumab and infliximab have the capability of lysing cells involved in the inflammatory process, whereas the receptor fusion protein apparently lacks this capability.[37] Although the clinical significance of these differences have not been absolutely proven, etanercept, has been shown to perform worse than placebo for Crohn's disease. These differences may account for the differential actions of these drugs in both efficacy and side effects.[medical citation needed]
Infliximab has high specificity for TNF-α, and does not neutralize TNF beta (TNFβ, also called lymphotoxin α), an unrelated cytokine that uses different receptors from TNF-α. Biological activities attributed to TNF-α include induction of proinflammatory cytokines (such as interleukins IL-1 and IL-6), enhancement of leukocyte movement or migration from the blood vessels into the tissues (by increasing the permeability of endothelial layer of blood vessels), and increasing the release of adhesion molecules. Infliximab prevents disease in transgenic mice (a special type of mice biologically engineered to produce a human form of TNF-α and which are used to test the results of these drugs that might be expected in humans). These experimental mice develop arthritis as a result of their production of human TNF-α, and when administered after disease onset, infliximab allows eroded joints to heal.[medical citation needed]
Other monoclonal antibodies targeting TNF-α are golimumab, adalimumab, and certolizumab pegol. Etanercept also binds and inhibits the action of TNF-α, but is not a monoclonal antibody (it is instead a fusion of TNF-receptor and an antibody constant region).[38]
History
Infliximab was originally developed in mice as a mouse antibody. Because humans have immune reactions to mouse proteins, the mouse common domains were replaced with similar human antibody domains. They are monoclonal antibodies and have identical structures and affinities to the target. Because they are a combination of mouse and human antibody amino acid sequences, they are called a "chimeric monoclonal antibody".[medical citation needed]
The role of TNF as a key player in the development of rheumatoid arthritis was originally demonstrated by George Kollias and colleagues in proof of principle studies in transgenic animal models.[39][40]
Infliximab was developed by Junming Le (b.1940) and Jan Vilček (b.1933) at New York University School of Medicine and in collaboration with Centocor, (now Janssen Biotech, Inc.)[41]
Society and culture
Marketing
Remicade is marketed by Janssen Biotech, Inc. (formerly Centocor Biotech, Inc.) in the United States, Mitsubishi Tanabe Pharma in Japan, Xian Janssen in China, and Schering-Plough (now part of Merck & Co) elsewhere.[42]
Biosimilars
In June 2013, two biosimilar versions (Inflectra and Remsima) were submitted for approval in Europe, by Hospira and Celltrion Healthcare respectively.[43] Both had a positive opinion from European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) for sale in the European Union (EU).[44] Celltrion obtained marketing authorization approval (MAA) from 27 EU countries and 3 EEA (European Economic Area) countries by September 2013.[45][46][47] Inflectra was approved for use in the European Union in September 2013,[47] and Remsima was approved for use in the European Union in October 2013.[46]
In Japan, Celltrion received marketing authorization for Remsima from Japan's Ministry of Health, Labour and Welfare (MHLW) in July 2014.[citation needed]
In India, Epirus Biopharmaceuticals obtained approval to produce biosimilar infliximab under the brand name "Infimab" (trail name BOW015).[48]
The U.S. Food and Drug Administration (FDA) approved Celltrion/Hospira/Pfizer's Inflectra (infliximab-dyyb) in April 2016.[9][49] Although the US brand name is Inflectra, it is marketed in the European Union as Remsima.[46]
The FDA approved Samsung Bioepis Co., Ltd.'s Renflexis (infliximab-abda) in April 2017.[50]
Biogen released another biosimilar, Flixabi, which was approved in Germany, the UK, and the Netherlands.[51] Flixabi was approved for use in the European Union in May 2016.[52]
In December 2017, Ixifi (infliximab-qbtx) was approved in the United States.[53]
Zessly was approved for use in the European Union in May 2018.[54]
In December 2019, Avsola (infliximab-axxq) was approved in the United States.[55]
Availability/affordability
In the United States, Infliximab is an expensive medication, costing about US$900 for a 100 mg dose, and is covered by almost every US medical insurance plan (though caps on many plans make it possible to be covered for only a subset of treatments in the course of a year).[citation needed] Infliximab is supplied as a sterile, white, lyophilized (freeze-dried) powder,[56] so must be reconstituted and administered by a health care professional, usually in a hospital or office setting.[42] For this reason, it is usually covered under major medical insurance rather than prescription drug coverage. The loading regimen for all approved indications occurs at weeks 0, 2, and 6 at the above dosages.[42]
In the UK, Infliximab is available from the NHS for Crohn's disease treatment provided three criteria are met.[57] Patients should have severe active Crohn's disease with a CDAI score of 300 or more, have not responded to immunomodulating drugs and corticosteroids, and for whom surgery is inappropriate. Since February 2015, it is also approved for the treatment of ulcerative colitis where other treatments have not worked.[58]
In Australia, Infliximab is available through the PBS for Crohn's disease treatment provided the patient has not responded to conventional treatment and is suffering from a severe case of the condition.[59]
Infliximab is available in the Republic of Ireland through the HSE's Medical Card and Drug Payment Scheme.[citation needed]
Johnson & Johnson reported in its 2013 annual report, "Remicade (infliximab), accounted for approximately 9.4% of the Company's total revenues for fiscal 2013." [60]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Infliximab, Infliximab-dyyb Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 15 July 2019. Retrieved 15 July 2019.
- ↑ 2.0 2.1 "Infliximab Use During Pregnancy". Drugs.com. 2 July 2019. Archived from the original on 30 November 2020. Retrieved 13 August 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 1153. ISBN 9780857113658.
- ↑ "Infliximab Use During Pregnancy". Drugs.com. Archived from the original on 30 November 2020. Retrieved 29 October 2020.
- ↑ "Remicade EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 22 October 2020. Retrieved 2 April 2020.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "EC approves first monoclonal antibody biosimilar / News / Biosimilars / Home - GaBI Online - Generics and Biosimilars Initiative". www.gabionline.net. Archived from the original on 24 October 2020. Retrieved 29 October 2020.
- ↑ "Nippon Kayaku launches biosimilar Remicade in Japan". www.thepharmaletter.com. Archived from the original on 13 October 2015. Retrieved 29 October 2020.
- ↑ 9.0 9.1 "FDA approves Inflectra, a biosimilar to Remicade". U.S. Food and Drug Administration (FDA) (Press release). 6 December 2019. Archived from the original on 7 December 2019. Retrieved 6 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Infliximab-Axxq Prices and Infliximab-Axxq Coupons". GoodRx. Retrieved 29 October 2020.
- ↑ Ton, Joey (27 May 2019). "#236 It's all in the details... or is it? Biosimilars versus biologics for inflammatory conditions". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 15 June 2023.
- ↑ Dubinsky MC, Fleshner PP (June 2003). "Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes". Curr Treat Options Gastroenterol. 6 (3): 183–200. doi:10.1007/s11938-003-0001-1. PMID 12744819. S2CID 21302609.
- ↑ Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ (May 1999). "Infliximab for the treatment of fistulas in patients with Crohn's disease" (PDF). The New England Journal of Medicine. 340 (18): 1398–405. doi:10.1056/NEJM199905063401804. PMID 10228190. Archived (PDF) from the original on 18 February 2019. Retrieved 5 July 2019.
- ↑ Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ (February 2004). "Infliximab maintenance therapy for fistulizing Crohn's disease". The New England Journal of Medicine. 350 (9): 876–85. doi:10.1056/NEJMoa030815. PMID 14985485.
- ↑ 15.0 15.1 Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P (May 2002). "Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial". Lancet. 359 (9317): 1541–9. doi:10.1016/S0140-6736(02)08512-4. PMID 12047962. S2CID 1905194.
- ↑ Hanauer SB (February 2003). "Crohn's disease: step up or top down therapy". Best Pract Res Clin Gastroenterol. 17 (1): 131–7. doi:10.1053/bega.2003.0361. PMID 12617888.
- ↑ Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF (2005). "Infliximab for induction and maintenance therapy for ulcerative colitis". The New England Journal of Medicine. 353 (23): 2462–76. doi:10.1056/NEJMoa050516. PMID 16339095.
- ↑ Motaghi, Ehsan; Ghasemi-Pirbaluti, Masoumeh; Zabihi, Mohsen (January 2019). "Etrolizumab versus infliximab in the treatment of induction phase of ulcerative colitis: A systematic review and indirect comparison". Pharmacological Research. 139: 120–125. doi:10.1016/j.phrs.2018.11.003. PMID 30395950. S2CID 53235342.
- ↑ Kavanaugh AF, Ritchlin CT (July 2006). "Systematic review of treatments for psoriatic arthritis: an evidence based approach and basis for treatment guidelines". J. Rheumatol. 33 (7): 1417–21. PMID 16724373.
- ↑ 20.0 20.1 Maxwell, LJ; Zochling, J; Boonen, A; Singh, JA; Veras, MM; Tanjong Ghogomu, E; Benkhalti Jandu, M; Tugwell, P; Wells, GA (18 April 2015). "TNF-alpha inhibitors for ankylosing spondylitis". The Cochrane Database of Systematic Reviews. 4 (4): CD005468. doi:10.1002/14651858.CD005468.pub2. PMID 25887212. Archived from the original on 20 September 2020. Retrieved 11 December 2019.
- ↑ Blumenauer, Barbara BTB; Judd, Maria; Wells, George A; Burls, Amanda; Cranney, Ann; Hochberg, Marc C; Tugwell, Peter; Lopez-Olivo, Maria Angeles (2002-07-22). "Infliximab for the treatment of rheumatoid arthritis". Cochrane Database of Systematic Reviews (3): CD003785. doi:10.1002/14651858.cd003785. ISSN 1465-1858. PMID 12137712.
- ↑ Sfikakis PP (November 2002). "Behçet's disease: a new target for anti-tumour necrosis factor treatment". Ann. Rheum. Dis. 61 Suppl 2 (Suppl 2): ii51–3. doi:10.1136/ard.61.suppl_2.ii51. PMC 1766720. PMID 12379622.
- ↑ 23.0 23.1 Puéchal X, Terrier B, Mouthon L, Costedoat-Chalumeau N, Guillevin L, Le Jeunne C (March 2014). "Relapsing polychondritis". Joint, Bone, Spine : Revue du Rhumatisme. 81 (2): 118–24. doi:10.1016/j.jbspin.2014.01.001. PMID 24556284. Archived from the original on 5 November 2018. Retrieved 18 May 2018.
- ↑ Kemta Lekpa F, Kraus VB, Chevalier X (April 2012). "Biologics in relapsing polychondritis: a literature review". Seminars in Arthritis and Rheumatism. 41 (5): 712–9. doi:10.1016/j.semarthrit.2011.08.006. PMID 22071463.
- ↑ Gupta AK, Skinner AR (2004). "A review of the use of infliximab to manage cutaneous dermatoses". J Cutan Med Surg. 8 (2): 77–89. doi:10.1007/s10227-004-0115-7. PMID 15685387. S2CID 23838714.
- ↑ Wijsenbeek, MS; Culver, DA (December 2015). "Treatment of Sarcoidosis". Clinics in Chest Medicine. 36 (4): 751–67. doi:10.1016/j.ccm.2015.08.015. PMID 26593147.
- ↑ Rennard SI, Fogarty C, Kelsen S, Long W, Ramsdell J, Allison J, Mahler D, Saadeh C, Siler T, Snell P, Korenblat P, Smith W, Kaye M, Mandel M, Andrews C, Prabhu R, Donohue JF, Watt R, Lo KH, Schlenker-Herceg R, Barnathan ES, Murray J (May 2007). "The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease". Am. J. Respir. Crit. Care Med. 175 (9): 926–34. doi:10.1164/rccm.200607-995OC. PMID 17290043.
- ↑ Brahmer, Julie R.; Lacchetti, Christina; Schneider, Bryan J.; Atkins, Michael B.; Brassil, Kelly J.; Caterino, Jeffrey M.; Chau, Ian; Ernstoff, Marc S.; Gardner, Jennifer M.; Ginex, Pamela; Hallmeyer, Sigrun; Holter Chakrabarty, Jennifer; Leighl, Natasha B.; Mammen, Jennifer S.; McDermott, David F.; Naing, Aung; Nastoupil, Loretta J.; Phillips, Tanyanika; Porter, Laura D.; Puzanov, Igor; Reichner, Cristina A.; Santomasso, Bianca D.; Seigel, Carole; Spira, Alexander; Suarez-Almazor, Maria E.; Wang, Yinghong; Weber, Jeffrey S.; Wolchok, Jedd D.; Thompson, John A. (10 June 2018). "Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline". Journal of Clinical Oncology. 36 (17): 1714–1768. doi:10.1200/JCO.2017.77.6385. PMC 6481621. PMID 29442540.
- ↑ Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM (2001). "Tuberculosis associated with infliximab, a tumor necrosis factor α–neutralizing agent". N Engl J Med. 345 (15): 1098–1104. doi:10.1056/NEJMoa011110. PMID 11596589.
- ↑ "Remicade for Healthcare Professionals". Remicade. Archived from the original on 2008-07-04.
- ↑ [1] Archived October 9, 2006, at the Wayback Machine
- ↑ "Tumor Necrosis Factor (TNF) Blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi) August 2009". MedWatch. U.S. Food and Drug Administration (FDA). August 31, 2009. Archived from the original on November 15, 2009. Retrieved 2009-11-15.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P (Dec 4, 1999). "Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group". Lancet. 354 (9194): 1932–9. doi:10.1016/s0140-6736(99)05246-0. PMID 10622295. S2CID 104011.
- ↑ 34.0 34.1 Akiho, Hirotada; Yokoyama, Azusa; Abe, Shuichi; Nakazono, Yuichi; Murakami, Masatoshi; Otsuka, Yoshihiro; Fukawa, Kyoko; Esaki, Mitsuru; Niina, Yusuke (2015-11-15). "Promising biological therapies for ulcerative colitis: A review of the literature". World Journal of Gastrointestinal Pathophysiology. 6 (4): 219–227. doi:10.4291/wjgp.v6.i4.219. ISSN 2150-5330. PMC 4644886. PMID 26600980.
- ↑ Choy EH, Panayi GS (March 2001). "Cytokine pathways and joint inflammation in rheumatoid arthritis". The New England Journal of Medicine. 344 (12): 907–16. doi:10.1056/NEJM200103223441207. PMID 11259725.
- ↑ Etanercept product labeling
- ↑ Etanercept, Adalimumab and Infliximab product labeling
- ↑ Peppel K, Crawford D, Beutler B (1991). "A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity". J. Exp. Med. 174 (6): 1483–9. doi:10.1084/jem.174.6.1483. PMC 2119031. PMID 1660525.
- ↑ Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G (December 1991). "Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis". The EMBO Journal. 10 (13): 4025–31. doi:10.1002/j.1460-2075.1991.tb04978.x. PMC 453150. PMID 1721867.
- ↑ Brenner D, Blaser H, Mak TW (May 2015). "Regulation of tumour necrosis factor signalling: live or let die". Nat Rev Immunol. 15 (6): 362–74. doi:10.1038/nri3834. PMID 26008591. S2CID 1550839.
- ↑ Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P (November 1993). "Construction and initial characterization of a mouse-human chimeric anti-TNF antibody". Mol. Immunol. 30 (16): 1443–53. doi:10.1016/0161-5890(93)90106-L. PMID 8232330.
- ↑ 42.0 42.1 42.2 "Remicade Becomes First Anti-TNF Biologic Therapy to Treat One Million Patients Worldwide" (Press release). Johnson & Johnson. November 6, 2007. Archived from the original on July 13, 2011. Retrieved 2009-11-14.
- ↑ George, John (June 28, 2013). "Billion-dollar biotech drug may soon have biosimilar competition". Philadelphia Business Journal. Archived from the original on July 1, 2013. Retrieved June 27, 2013.
- ↑ "European Medicines Agency recommends approval of first two monoclonal-antibody biosimilars" Archived 2015-02-17 at the Wayback Machine, European Medicines Agency, June 28, 2013
- ↑ Rockoff, Jonathan D. (10 September 2013). "European Commission Approves Biosimilar of J&J and Merck's Remicade". The Wall Street Journal. Archived from the original on 2017-01-25. Retrieved 1 March 2020.
- ↑ 46.0 46.1 46.2 "Remsima EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 29 December 2019. Retrieved 1 March 2020.
- ↑ 47.0 47.1 "Inflectra EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 16 October 2019. Retrieved 1 March 2020.
- ↑ Serebrov, Mari. "Epirus racks up its first biosimilar approval in India". BioWorld. Archived from the original on 29 October 2014. Retrieved 26 October 2014.
- ↑ "Inflectra: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). 6 December 2019. Archived from the original on 7 December 2019. Retrieved 6 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Renflexis: FDA Approved Drug Products". U.S. Food and Drug Administration (FDA). 17 April 2019. Archived from the original on 17 April 2019. Retrieved 6 December 2019.
{{cite web}}: CS1 maint: unfit URL (link) This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Key Takeaways from Biogen's Q3 Call: Tecfidera, Pipeline, Biosimilars". nasdaq.com. 27 October 2016. Archived from the original on 28 October 2016.
- ↑ "Flixabi EPAR". European Medicines Agency (EMA). Archived from the original on 16 October 2019. Retrieved 2 April 2020.
- ↑ "Ixifi: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). 6 December 2019. Archived from the original on 7 December 2019. Retrieved 6 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Zessly EPAR". European Medicines Agency (EMA). Archived from the original on 30 December 2019. Retrieved 2 April 2020.
- ↑ "Avsola: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). 6 December 2019. Archived from the original on 7 December 2019. Retrieved 6 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Remicade (Infliximab): Side Effects, Interactions, Warning, Dosage & Uses". rxlist.com. Archived from the original on 2009-12-18.
- ↑ "The clinical effectiveness and cost effectiveness of infliximab for Crohn's disease - Guidance and guidelines - NICE". guidance.nice.org.uk. Archived from the original on 2010-04-30.
- ↑ "Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy". National Institute for Health and Care Excellence (NICE). Archived from the original on 2015-03-06.
- ↑ "Section 100 arrangements; only for infliximab". medicareaustralia.gov.au. Archived from the original on 3 September 2011. Retrieved 20 August 2011.
- ↑ JNJ annual report, downloaded April 22, 2014. "Archived copy". Archived from the original on 2016-03-05. Retrieved 2017-09-01.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- CS1: long volume value
- Webarchive template wayback links
- CS1 maint: unfit URL
- CS1 maint: archived copy as title
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugs that are a monoclonal antibody
- All articles with unsourced statements
- Articles with unsourced statements from December 2019
- Articles with invalid date parameter in template
- Articles with unsourced statements from August 2020
- Articles with unsourced statements from March 2020
- Articles with unsourced statements from March 2009
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed EBI identifier
- Engineered proteins
- Immunosuppressants
- Johnson & Johnson brands
- Janssen Biotech
- Janssen Pharmaceutica
- Merck & Co. brands
- Monoclonal antibodies
- Schering-Plough brands
- TNF inhibitors
- World Health Organization essential medicines (alternatives)
- RTT