Relugolix
 | |
 | |
| Names | |
|---|---|
| Trade names | Orgovyx, Relumina |
| Other names | RGX; RVT-601; TAK-385 |
| |
| Clinical data | |
| Drug class | GnRH antagonist |
| Main uses | Prostate cancer, uterine fibroids[1][2] |
| Side effects | Hot flashes, increase blood sugar, muscle pain, low hemoglobin, tiredness, constipation[1] |
| Routes of use | By mouth[3] |
| Typical dose | 120 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 68–71%[3] |
| Elimination half-life | 36 to 65 hours[3] |
| Excretion | Feces: 82%[3] Urine: 4%[3] |
| Chemical and physical data | |
| Formula | C29H27F2N7O5S |
| Molar mass | 623.64 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Relugolix, sold under the brand names Orgovyx and Relumina, is a medication used to treat prostate cancer and uterine fibroids.[1][2] In prostate cancer it is used for cases that are advanced but hormone sensitive.[4] It is taken by mouth once per day.[1]
Common side effects include hot flashes, increase blood sugar, muscle pain, low hemoglobin, tiredness, and constipation.[1] Other side effects may include QT prolongation.[1] Use in pregnancy, by either partner, may harm the baby.[1] It is a gonadotropin-releasing hormone (GnRH) receptor antagonist, which blocks the production of testosterone.[1][4]
Relugolix was approved for medical use in Japan in 2019, the United States in 2020, and Europe in 2022.[1][2][4] In the United States a month of treatment costs about 2,450 USD per month as of 2022.[5] It is being studied in endometriosis.[6] It is also one component of relugolix/estradiol/norethisterone.[7]
Medical uses
Relugolix is approved in the United States for the treatment of prostate cancer and in Japan for the treatment of uterine fibroids (uterine leiomyoma).[8][9][1] It is used at a dosage of 120 mg once daily by mouth in the treatment of prostate cancer (after a single 360 mg loading dose on the first day of therapy) and at a dosage of 40 mg once daily in the treatment of uterine fibroids.[3][1]
Available forms
Relugolix is available in the form of 40 and 120 mg oral tablets.[1][10][9]
Dosage
For prostate cancer it is started at a dose of 360 mg followed by 120 mg per day.[4][1]
Side effects
The main side effects of relugolix for uterine fibroids include abnormal uterine bleeding (24.6–48.6% vs. 6.3% for placebo), hot flashes (42.8–45.5% vs. 0% for placebo), heavy menstrual bleeding (12.1–49.3% vs. 9.4% for placebo), headache (12.3–15.2%), and excessive sweating (9.4–15.2% vs. 0% for placebo).[3][9] In addition, decreased bone mineral density occurs with relugolix (21.7% decrease by week 12, 24.4% decrease by week 24).[3]
Pharmacology
Pharmacodynamics
Relugolix is a selective antagonist of the gonadotropin-releasing hormone receptor (GnRHR), with a half-maximal inhibitory concentration (IC50) of 0.12 nM.[3][11][12]
A dosage of relugolix of 40 mg once per day has been found to suppress estradiol levels to postmenopausal levels (<20 pg/mL) within 24 hours in premenopausal women.[3] In the control group of women, estradiol levels fluctuated between 50 and 250 pg/mL.[3] Estradiol levels have been found to return to normal concentrations within 4 weeks of discontinuation of relugolix in premenopausal women.[3] The medication additionally suppresses levels of progesterone, luteinizing hormone, and follicle-stimulating hormone in premenopausal women.[3] Relugolix at a dosage of 40 mg or more once per day has been found to reduce testosterone levels to sustained castrate levels (<20 ng/dL) in men.[13] It additionally suppresses luteinizing hormone and follicle-stimulating hormone levels in men.[13]
Lower doses of relugolix (<40 mg/day) are under investigation for achieving partial sex hormone suppression in the treatment of endometriosis and uterine fibroids.[14] This is intended to reduce the incidence and severity of menopausal symptoms such as hot flushes and decreased bone mineral density that are secondary to estrogen deficiency.[14][15]
Pharmacokinetics
A single 40-mg oral dose of relugolix has been found to result in peak levels of relugolix of 29 ng/mL (47 nmol/L) after 1.5 hours.[3] Steady-state levels are reached within 7 days with 40 mg/day relugolix administration.[3] There is an approximate 2-fold accumulation of relugolix by 2 weeks of continuous administration.[3] Food diminishes the oral bioavailability of relugolix by about 50%.[3]
Relugolix is a substrate for P-glycoprotein, which may have a limiting effect on its absorption and distribution.[3] The plasma protein binding of relugolix is approximately 68 to 71% over a concentration range of 0.05 to 5 μg/mL.[3]
Relugolix is not a substrate for CYP3A4.[3] The elimination half-life of relugolix is 36 to 65 hours across a dosage range of 20 to 180 mg/day.[3] There is moderate to high interindividual variability in systemic exposure to relugolix.[3]
Relugolix is excreted mainly in feces (83%) and to a small degree in urine (4%).[3] Only about 6% of a dose of relugolix is excreted unchanged.[3]
-
Mechanism of action[16]
-
Estradiol levels with 40 mg relugolix once per day in premenopausal women relative to untreated premenopausal women.[9]
Chemistry
Relugolix is a non-peptide, small-molecule compound, and is structurally distinct from GnRH analogues.[17] It is an N-phenylurea derivative.[3]
History
Relugolix was first described in 2004.[19][11] It superseded sufugolix (developmental code name TAK-013), which was developed by the same researchers.[11] Relugolix was approved for the treatment of uterine fibroids in Japan on 8 January 2019.[8][10]
It was the second orally active GnRH antagonist to be introduced for medical use, following elagolix (brand name Orilissa) in July 2018.[8][20] Relugolix was approved for the treatment of prostate cancer in the United States on 18 December 2020.[8][1]
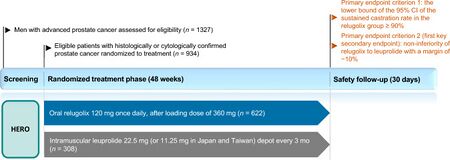
The FDA approved relugolix based on evidence from a clinical trial (NCT03085095) of 930 participants 48 to 97 years old with advanced prostate cancer.[21] The trial was conducted at 155 sites in the United States, Canada, and countries in South America, Europe and the Asia Pacific region.[21] All participants in the trial had advanced prostate cancer.[21] Participants were randomly assigned to receive either one relugolix tablet daily (on the first day they received three tables) or an active control (leuprolide acetate) which was given as an injection under the skin every three months.[21] The participants and healthcare providers were aware of which treatment was being given.[21] The treatment lasted for 48 weeks.[21] The efficacy of relugolix was assessed by the percentage of participants who achieved and maintained low testosterone level equal to castration.[21]
Society and culture
Names
Relugolix is the generic name of the drug and its INN, USAN, and JAN.[22][23] It is also known by its former developmental code names RVT-601 and TAK-385.[8][22]
Relugolix is sold under the brand name Orgovyx for the treatment of prostate cancer and under the brand name Relumina for the treatment of uterine fibroids.[8][10][9][1] Relugolix compounded with ethinyl estradiol and norethindrone is sold under the brand name "Myfembree" for the treatment of uterine fibroids.
Availability
Relugolix is available in the United States and in Japan.[1][10][9]
Legal status
On 24 February 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Orgovyx, intended for the treatment of prostate cancer.[24] The applicant for this medicinal product is Myovant Sciences Ireland Limited.[24]
Research
Relugolix is under development by Myovant Sciences and Takeda for the treatment of uterine fibroids in countries besides Japan such as the United States.[8] Relugolix is also under development for the treatment of endometriosis in the United States and other countries.[8] As of February 2019, relugolix is in phase III clinical trials for endometriosis.[8]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 "Orgovyx- relugolix tablet, film coated". DailyMed. Archived from the original on 26 May 2021. Retrieved 25 May 2021.
- ↑ 2.0 2.1 2.2 Markham, Anthony (April 2019). "Relugolix: First Global Approval". Drugs. 79 (6): 675–679. doi:10.1007/s40265-019-01105-0. ISSN 0012-6667. PMID 30937733. S2CID 89616869.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 Barra F, Seca M, Della Corte L, Giampaolino P, Ferrero S (August 2019). "Relugolix for the treatment of uterine fibroids". Drugs Today. 55 (8): 503–512. doi:10.1358/dot.2019.55.8.3020179. PMID 31461087. S2CID 201654739.
- ↑ 4.0 4.1 4.2 4.3 "Orgovyx". EMA. Archived from the original on 14 October 2022. Retrieved 2 November 2022.
- ↑ "Orgovyx". Retrieved 2 November 2022.
- ↑ Goenka L, George M, Sen M (June 2017). "A peek into the drug development scenario of endometriosis - A systematic review". Biomed. Pharmacother. 90: 575–585. doi:10.1016/j.biopha.2017.03.092. PMID 28407578.
- ↑ "Relugolix + estradiol + norethisterone". SPS - Specialist Pharmacy Service. 13 March 2017. Archived from the original on 3 March 2022. Retrieved 2 November 2022.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 "Relugolix - Myovant/Takeda - AdisInsight". Archived from the original on 2018-09-20. Retrieved 2022-04-14.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 "Relumina (relugolix) Information - ASKA Pharmaceutical" (PDF) (in 日本語). ASKA Pharmaceutical. January 2019. Archived from the original (PDF) on 2019-02-16. Retrieved 16 February 2019.
- ↑ 10.0 10.1 10.2 10.3 "Myovant Provides Corporate Updates and Reports Financial Results for Third Fiscal Quarter Ended December 31, 2018". Archived from the original on June 26, 2019. Retrieved April 14, 2022.
- ↑ 11.0 11.1 11.2 Miwa K, Hitaka T, Imada T, Sasaki S, Yoshimatsu M, Kusaka M, Tanaka A, Nakata D, Furuya S, Endo S, Hamamura K, Kitazaki T (July 2011). "Discovery of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor". J. Med. Chem. 54 (14): 4998–5012. doi:10.1021/jm200216q. PMID 21657270.
- ↑ Nakata D, Masaki T, Tanaka A, Yoshimatsu M, Akinaga Y, Asada M, Sasada R, Takeyama M, Miwa K, Watanabe T, Kusaka M (January 2014). "Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice". Eur. J. Pharmacol. 723: 167–74. doi:10.1016/j.ejphar.2013.12.001. PMID 24333551.
- ↑ 13.0 13.1 MacLean DB, Shi H, Faessel HM, Saad F (December 2015). "Medical Castration Using the Investigational Oral GnRH Antagonist TAK-385 (Relugolix): Phase 1 Study in Healthy Males". J. Clin. Endocrinol. Metab. 100 (12): 4579–87. doi:10.1210/jc.2015-2770. PMC 4667159. PMID 26502357.
- ↑ 14.0 14.1 Streuli I, de Ziegler D, Borghese B, Santulli P, Batteux F, Chapron C (March 2012). "New treatment strategies and emerging drugs in endometriosis". Expert Opin Emerg Drugs. 17: 83–104. doi:10.1517/14728214.2012.668885. PMID 22439891. S2CID 27472695.
- ↑ Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, Bozigian HP (February 2009). "Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix". J. Clin. Endocrinol. Metab. 94 (2): 545–51. doi:10.1210/jc.2008-1695. PMC 2646513. PMID 19033369.
- ↑ Tatenuma, Tomoyuki; Miyamoto, Hiroshi (4 August 2023). "Profile of Relugolix in the Management of Advanced Hormone-Sensitive Prostate Cancer: Design, Development, and Place in Therapy". Drug Design, Development and Therapy. 17: 2325–2333. doi:10.2147/DDDT.S373546. Archived from the original on 5 August 2023. Retrieved 5 April 2024.
- ↑ Tukun FL, Olberg DE, Riss PJ, Haraldsen I, Kaass A, Klaveness J (December 2017). "Recent Development of Non-Peptide GnRH Antagonists". Molecules. 22 (12): 2188. doi:10.3390/molecules22122188. PMC 6149776. PMID 29232843.
- ↑ Shirley, Matt (2023). "Relugolix: A Review in Advanced Prostate Cancer". Targeted Oncology. 18 (2): 295–302. doi:10.1007/s11523-022-00944-4. ISSN 1776-2596. Retrieved 5 March 2024.
- ↑ "Thienopyrimidine compounds and use thereof". Archived from the original on 2022-01-09. Retrieved 2022-04-14.
- ↑ "Elagolix - Abbvie/Neurocrine Biosciences - AdisInsight". Archived from the original on 2018-09-20. Retrieved 2022-04-14.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 "Drug Trial Snapshot: Orgovyx". U.S. Food and Drug Administration (FDA). 18 December 2020. Archived from the original on 6 January 2021. Retrieved 6 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 22.0 22.1 "ChemIDplus - 737789-87-6 - AOMXMOCNKJTRQP-UHFFFAOYSA-N - Relugolix [USAN:INN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". Archived from the original on 2022-02-17. Retrieved 2022-04-14.
- ↑ "KEGG DRUG: Relugolix". Archived from the original on 2021-11-29. Retrieved 2022-04-14.
- ↑ 24.0 24.1 "Orgovyx: Pending EC decision". European Medicines Agency. 24 February 2022. Archived from the original on 27 February 2022. Retrieved 27 February 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
Further reading
- Elsharoud, A.; Ali, M.; Al-Hendy, A. (2019). "Relugolix. GnRH (LHRH) receptor antagonist, Treatment of uterine fibroids, Treatment of endometriosis-related pain, Treatment of prostate cancer". Drugs of the Future. 44 (2): 131. doi:10.1358/dof.2019.44.2.2927590. ISSN 0377-8282. S2CID 87369995.
- Barra F, Seca M, Della Corte L, Giampaolino P, Ferrero S (August 2019). "Relugolix for the treatment of uterine fibroids". Drugs Today. 55 (8): 503–512. doi:10.1358/dot.2019.55.8.3020179. PMID 31461087. S2CID 201654739.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Clinical trial number NCT03085095 for "A Study to Evaluate the Safety and Efficacy of Relugolix in Men With Advanced Prostate Cancer (HERO)" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- CS1 日本語-language sources (ja)
- Wikipedia articles incorporating the PD-notice template
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Dimethylamino compounds
- Ethers
- Fluoroarenes
- GnRH antagonists
- Hormonal antineoplastic drugs
- Pyridazines
- Pyrimidines
- Triketones
- Ureas
- RTT
![Mechanism of action[16]](https://nccommons.org/media/thumb/5/59/DDDT_A_373546_O_F0001g_%281%29.jpg/937px-DDDT_A_373546_O_F0001g_%281%29.jpg)
![Estradiol levels with 40 mg relugolix once per day in premenopausal women relative to untreated premenopausal women.[9]](https://upload.wikimedia.org/wikipedia/commons/thumb/e/e7/Estradiol_levels_with_40_mg_per_day_oral_relguolix_therapy_in_premenopausal_women.png/479px-Estradiol_levels_with_40_mg_per_day_oral_relguolix_therapy_in_premenopausal_women.png)